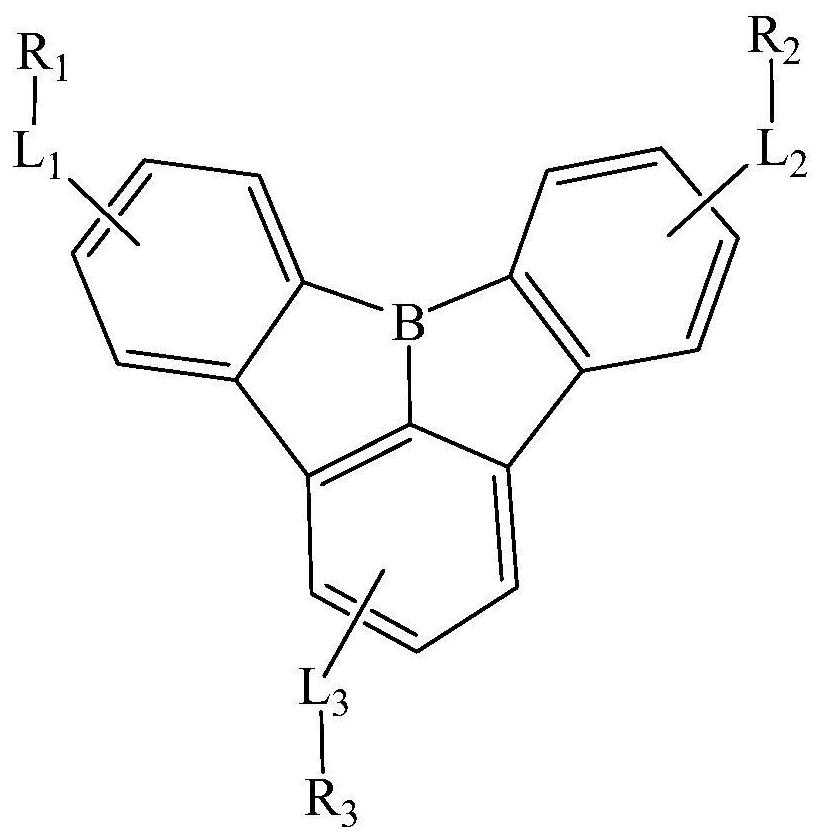
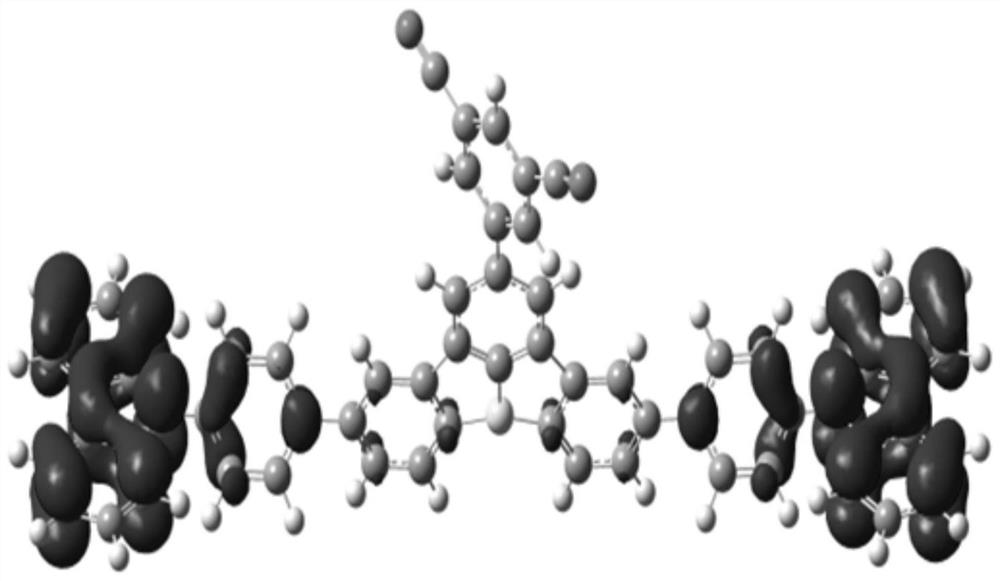
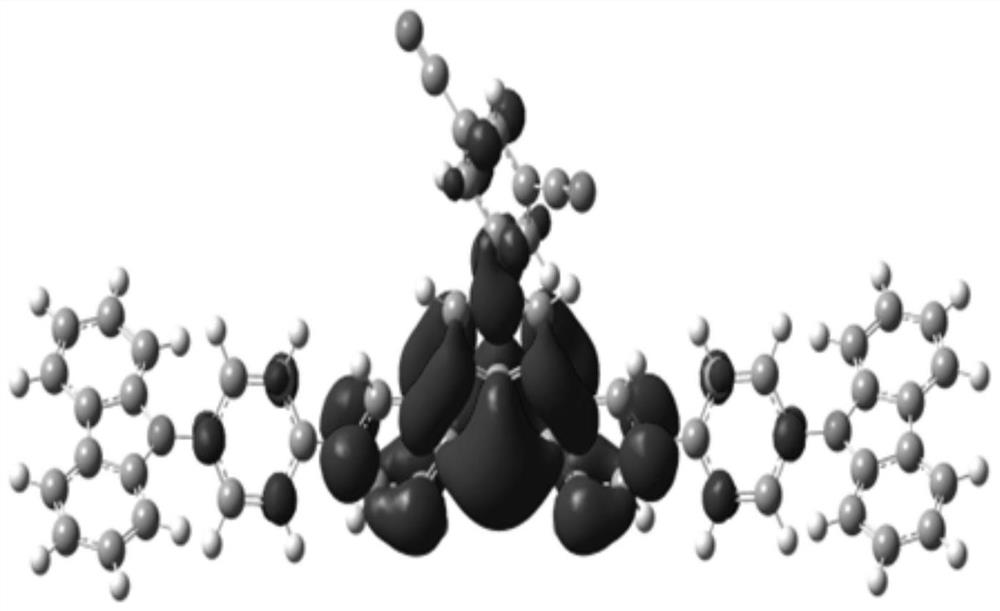
Compound, display panel and display device
A display panel and compound technology, applied in the field of organic electroluminescent materials, to achieve the effects of improving device efficiency, increasing luminous efficiency, and reducing overlap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Synthesis of Compound M1
[0110] Synthesis of Compound B
[0111]
[0112] In a 250ml three-neck flask, 9.40g (20mmol) of substrate A (20mmol) and THF (80mL) were added to dissolve, and nitrogen replacement was performed three times. Cool down to -78°C, and when the temperature reaches, control the temperature below -65°C and slowly add n-BuLi 20mL (50mmol) dropwise, and stir for 30min after the dropwise addition is complete. Then 4.35 g (40 mmol) of TMS-Cl was slowly added dropwise, and the temperature was raised to 0° C. for 4 h. After completion, add ice water to quench. DCM (80mL×2) was added for extraction. The collected organic phase was rotary evaporated to obtain an oil, which was crystallized using toluene / EtOH to obtain a solid. 9.12 g (20 mmol) of solid, anhydrous toluene solution (70 mL) and 2.5 g (10 mmol) of boron tribromide were sequentially added to a 200 mL pressure tube. Stir at 120°C for 12h. After the reaction H 2 O (100 mL) quenched. The...
Embodiment 2
[0141] Synthesis of compound M2
[0142] Synthesis of Compound B
[0143]
[0144] In a 250ml three-neck flask, 9.40g (20mmol) of substrate A (20mmol) and THF (80mL) were added to dissolve, and nitrogen replacement was performed three times. Cool down to -78°C, and when the temperature reaches, control the temperature below -65°C and slowly add n-BuLi 20mL (50mmol) dropwise, and stir for 30min after the dropwise addition is complete. Then 4.35 g (40 mmol) of TMS-Cl was slowly added dropwise, and the temperature was raised to 0° C. for 4 h. After completion, add ice water to quench. DCM (80mL×2) was added for extraction. The collected organic phase was rotary evaporated to obtain an oil, which was crystallized using toluene / EtOH to obtain a solid. 9.12 g (20 mmol) of solid, anhydrous toluene solution (70 mL) and 2.5 g (10 mmol) of boron tribromide were successively added into a 200 mL stuffy jar. Stir at 120°C for 12h. After the reaction H 2 O (100 mL) quenched. The ...
Embodiment 3
[0173] Synthesis of Compound M3
[0174] Synthesis of Compound B
[0175]
[0176] In a 250ml three-neck flask, 9.40g (20mmol) of substrate A (20mmol) and THF (80mL) were added to dissolve, and nitrogen replacement was performed three times. Cool down to -78°C, and when the temperature reaches, control the temperature below -65°C and slowly add n-BuLi 20mL (50mmol) dropwise, and stir for 30min after the dropwise addition is complete. Then 4.35 g (40 mmol) of TMS-Cl was slowly added dropwise, and the temperature was raised to 0° C. for 4 h. After completion, add ice water to quench. DCM (80mL×2) was added for extraction. The collected organic phase was rotary evaporated to obtain an oil, which was crystallized using toluene / EtOH to obtain a solid. 9.12 g (20 mmol) of solid, anhydrous toluene solution (70 mL) and 2.5 g (10 mmol) of boron tribromide were successively added into a 200 mL stuffy jar. Stir at 120°C for 12h. After the reaction H 2 O (100 mL) quenched. The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com