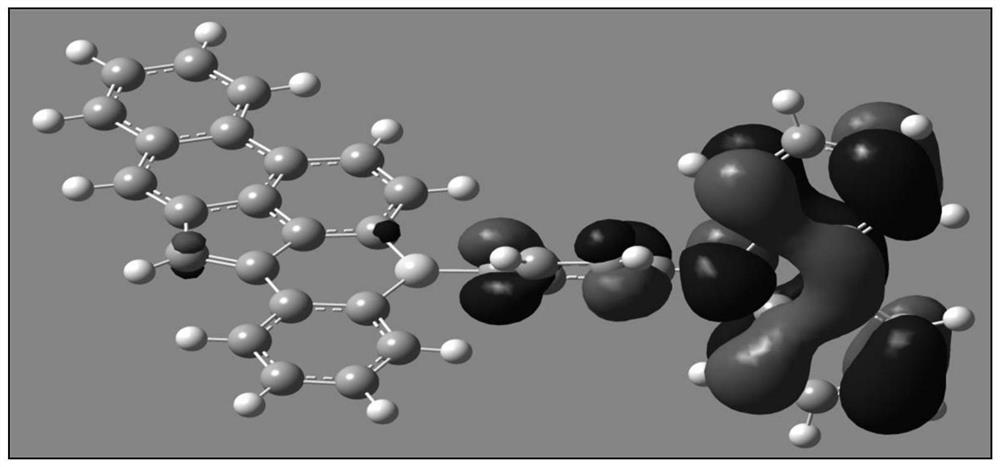
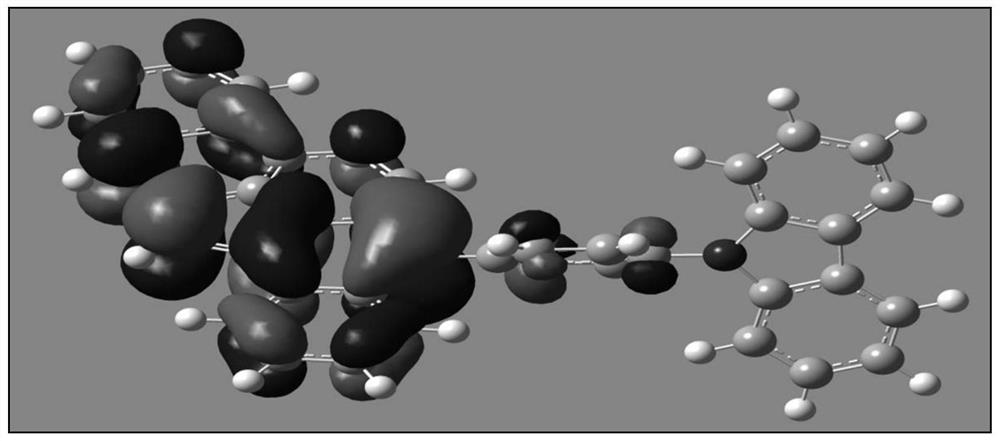
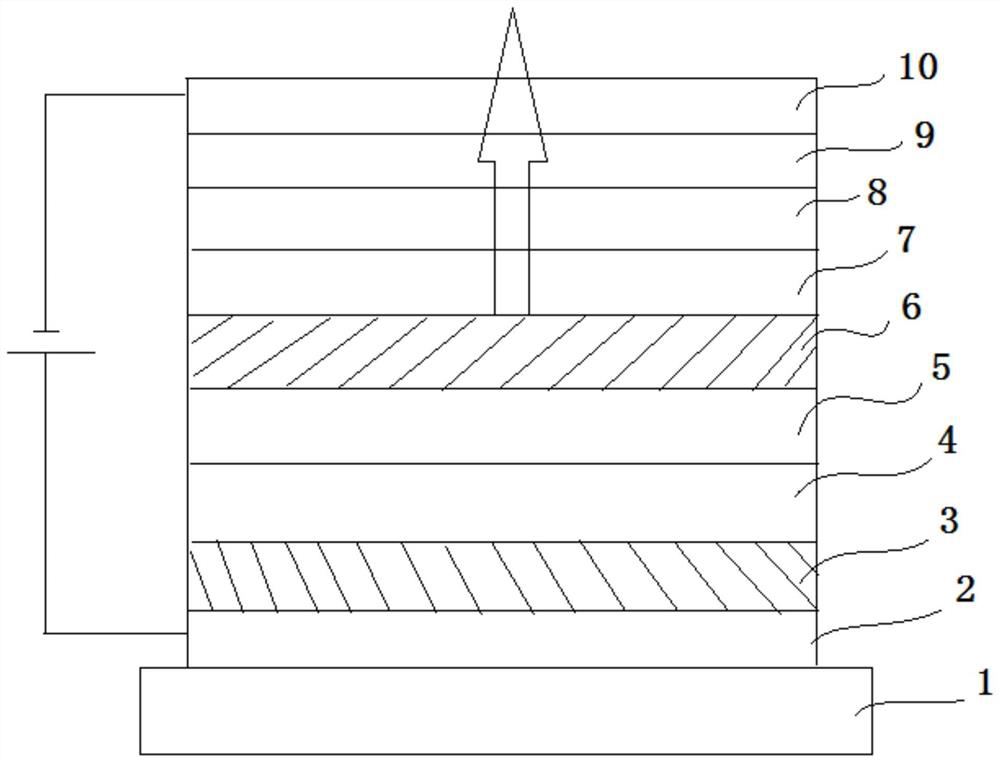
A boron-containing compound, display panel and display device
A boron compound and display panel technology, applied in the field of display panels and display devices, and boron-containing compounds, can solve the problems of high production costs, phosphorescent material efficiency roll-off, unfavorable mass production, etc., to improve device efficiency, reduce overlap, Enhanced effect of reverse crossing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062]
[0063] In a 250ml three-necked flask, add 3.56g (20mmol) of phenanthrene, that is, compound 1, compound 2, that is, 1.56g (20mmol) of benzene, and 1.78g (24mmol) of glyoxylic acid, mix and cool to 0°C, and then slowly add loaded trifluoro Methanesulfonic acid polyvinylpyrrolidone PVP-TfOH (3 g), the reaction mixture was warmed up to room temperature and stirred, and the reaction progress was monitored by TLC (hexane / ethyl acetate=3:1). After the reaction was complete, the mixture was poured into ice water, neutralized with saturated sodium bicarbonate, and extracted with ether (3×15 mL). The combined organic extracts were washed with water and washed with anhydrous Na 2 SO 4 After drying, the solvent was removed by evaporation in vacuo and the crude product was purified by column chromatography on silica gel using hexane / ethyl acetate as eluent. Add 75ml of thionyl chloride to the obtained product and mix coldly, heat the reaction mixture under reflux for 3 hours...
Embodiment 2
[0089]
[0090] Add compound 7 (1.95g) and diethyl ether (30mL) into a 250ml three-necked flask, replace with nitrogen three times, cool down to -78°C, control the temperature below -65°C and slowly add n-BuLi (0.34g) dropwise after the temperature reaches, After the dropwise addition was complete, stir for 30 minutes, then dissolve compound 8-2 (1.79 g) in 30 mL of toluene, slowly add it dropwise to the reaction solution, and naturally rise to room temperature for 6 hours after the dropwise addition, and add ice water (500 mL) to quench the reaction solution after the reaction. The reaction was quenched, and DCM (40mL*2) was added for extraction, and finally extracted once with saturated brine, and the collected organic phase was rotary evaporated to obtain a light yellow solid. 15 mL of toluene was added for thermal dissolution and crystallization to obtain compound M2.
[0091] 1 H NMR (400MHz, Chloroform) δ8.92 (d, J = 2.0Hz, 15H), 8.84 (s, 7H), 7.87 (t, J = 34.0Hz, 21...
Embodiment 3
[0094]
[0095] Add compound 7 (1.95g) and diethyl ether (30mL) into a 250ml three-necked flask, replace with nitrogen three times, cool down to -78°C, control the temperature below -65°C and slowly add n-BuLi (0.34g) dropwise after the temperature reaches, After the dropwise addition was complete, stir for 30min, then dissolve compound 8-3 (1.72g) in 30mL of toluene, slowly add dropwise to the reaction solution, after the dropwise addition, naturally rise to room temperature and react for 6h, after the reaction, add ice water (500mL) to quench The reaction was quenched, and DCM (40mL*2) was added for extraction, and finally extracted once with saturated brine, and the collected organic phase was rotary evaporated to obtain a light yellow solid. 15 mL of toluene was added for thermal dissolution and crystallization to obtain compound M3.
[0096] 1 H NMR (400MHz, Chloroform) δ9.15–8.82(m,35H),7.87(t,J=34.0Hz,43H),7.73(d,J=3.5Hz,7H),7.74–7.61(m,45H) ,7.50(s,8H),7.42(s,6H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com