A boron-containing compound, display panel and display device
A boron compound and display panel technology, applied in the fields of compounds containing group 3/13 elements of the periodic table, organic chemistry, chemical instruments and methods, etc., can solve the problem that the quantum yield can only reach 62.5%, and the stability of phosphorescent devices is not stable Good, phosphorescent material efficiency roll-off and other issues, to achieve the effect of low cost, reduce overlap, and improve luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
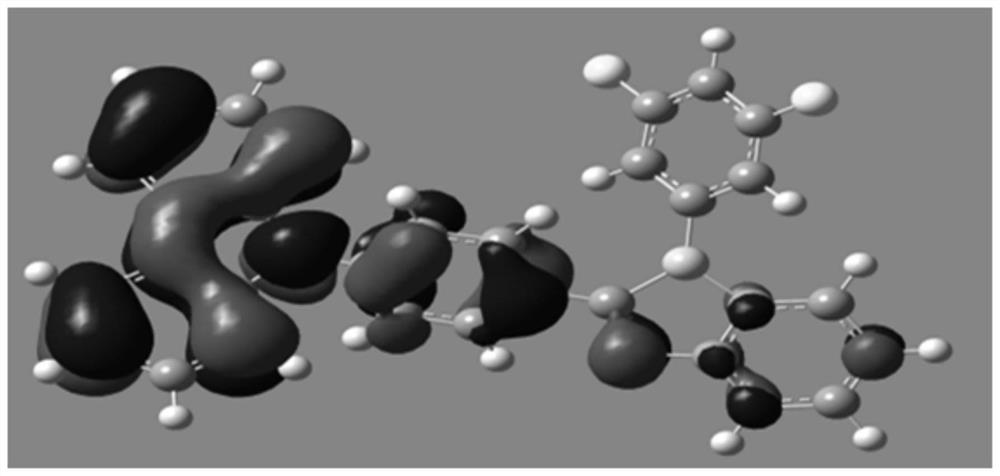
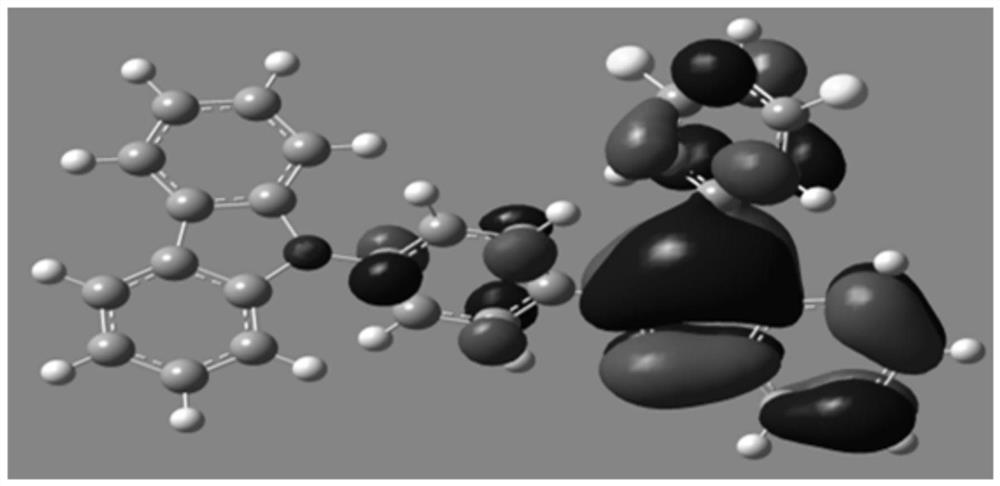
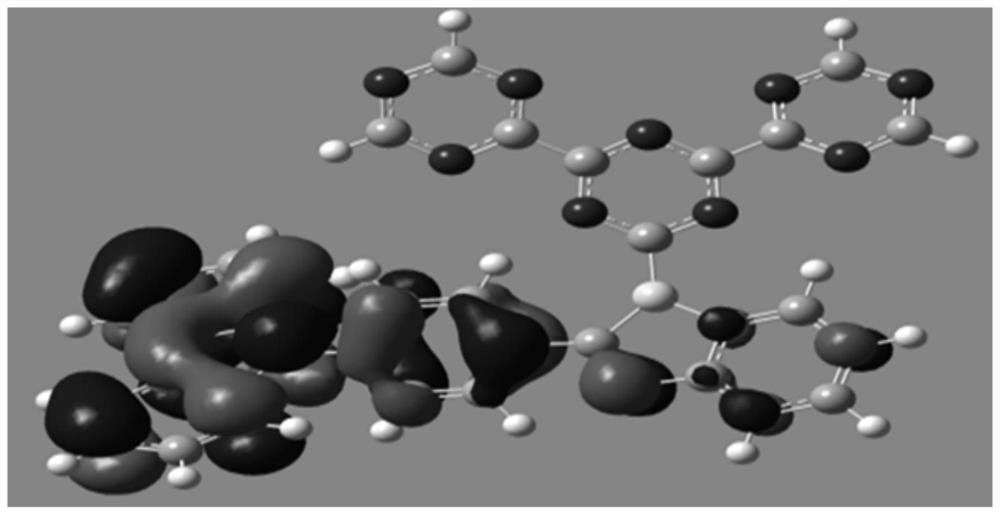
Image
Examples
Embodiment 1
[0073]
[0074] Add 6.84g (20mmol) of the compound dibromoimine-2-bromophenyl, 100ml THF and 1.2g magnesium strips sequentially into a 250ml three-necked flask, replace with nitrogen, heat to 50°C and stir for 30min. Dissolve 4.38g (20mmol) of the compound dimethyltin chloride in 60ml of toluene, and slowly add the solution dropwise to the reaction system. After the dropwise addition, it will naturally rise to room temperature and react for 6h. After the reaction is completed, add 60ml of ice water to quench the reaction. The reaction solution was extracted with DCM (100ml*3) and saturated brine (100ml*2) respectively, and the organic phase was rotary evaporated to obtain an oily product, which was recrystallized using TOL (toluene) / EtOH to obtain a solid compound 1.
[0075] MALDI-TOF: 330.90.
[0076] 1 H NMR (400MHz, Chloroform) δ7.42(s,1H),7.31(s,1H),7.16(s,1H),7.06(s,1H),1.44(s,6H).
[0077] 13 C NMR (100MHz, Common NMR Solvents) δ149.97(s), 144.21(s), 126.15(s), 12...
Embodiment 2
[0092]
[0093] Add 8.70 g (20 mmol) of compound 4 into the reaction flask, add diethyl ether (50 mL) to dissolve, and replace with nitrogen three times. Cool down to -78°C, and when the temperature reaches, control the temperature below -65°C and slowly add n-BuLi8.04mL (2.5M, 20mmol) dropwise, and stir for 30min after the dropwise addition is complete. Then 6.37 g (20 mmol) of compound 6 was dissolved in 60 mL of toluene, and slowly added dropwise to the reaction solution. After the dropwise addition was completed, it was naturally raised to room temperature for 6 h. After the reaction was completed, 100 mL of ice water was added to quench the reaction. Then add DCM (80mL*2) for extraction, and finally extract once with saturated brine. The collected organic phase was rotary evaporated, and the product was purified by column chromatography (mobile phase n-hexane:dichloromethane=3:1) to obtain compound M2.
[0094] MALDI-TOF: 593.05.
[0095] 1 H NMR (400MHz, Chlorofor...
Embodiment 3
[0099]
[0100] In a 250mL three-necked flask, 5.45g (20mmol) of compound 2, 8.43g (25mmol) of compound 7, 150mL of toluene, 9.21g (40mmol) of cesium carbonate, and 0.23g of tetrakis (triphenylphosphine) palladium ( 0.2 mmol) and 40.5 mg (0.2 mmol) of tri-tert-butylphosphine, and then reacted at 120° C. for 24 hours under a nitrogen atmosphere. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin dry the solvent and apply the sample by dry method, column chromatography (dichloromethane:n-hexane, v:v=1:1) After separation and purification, compound 8 was obtained.
[0101] MALDI-TOF: 484.01.
[0102] 1 H NMR (400MHz, Chloroform-d) δ8.12 (dd, J = 7.4, 1.6Hz, 2H), 8.00 (ddd, J = 7.2, 3.3, 1.4Hz, 2H), 7.89 (dd, J = 7.5, 1.5 Hz,1H),7.61–7.53(m,4H),7.50(td,J=7.5,1.5Hz,1H),7.44(t,J=7.4Hz,1H),7.40–7.22(m,6H),7.19 (dd,J=7.5,1.6Hz,1H).
[0103] 13 C NMR(100MHz,Chloroform-d)δ163.36,1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com