Nk1-antagonist combination and method for treating synucleinopathies
一种共核蛋白、拮抗剂的技术,应用在共核蛋白病领域,达到减缓症状进展、延迟发作的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0184] According to one embodiment, said 6-propylamino-4,5,6,7-tetrahydro-1,3-benzothiazol-2-amine is selected from:
[0185] -(S)-6-Propylamino-4,5,6,7-tetrahydro-1,3-benzothiazol-2-amine (INN: pramipexole) and its pharmaceutically acceptable salts, especially is its dihydrochloride monohydrate (USAN: pramipexole hydrochloride), the dosage / unit form is equivalent to 0.125mg to 45mg, preferably 0.125mg to 40-42mg pramipexole dihydrochloride monohydrate, with Equivalent to 0.375 mg to 45 mg, preferably greater than 6 mg to 45 mg or 6.5 mg to 45 mg, usually 0.375 mg to 40-42 mg, preferably greater than 6 mg to 40-42 mg or 6.5 mg to 40-42 mg pramipexole dihydrochloride monohydrate daily dose administration;
[0186] -(R,S)-6-Propylamino-4,5,6,7-tetrahydro-1,3-benzothiazol-2-amine (racemate) and its pharmaceutically acceptable salts, Dosage / unit form is from 0.25mg to 90mg, preferably greater than 12mg to 90mg, usually 0.25mg to 80mg, said dosage comprises the equivalent of 0.12...
Embodiment 1
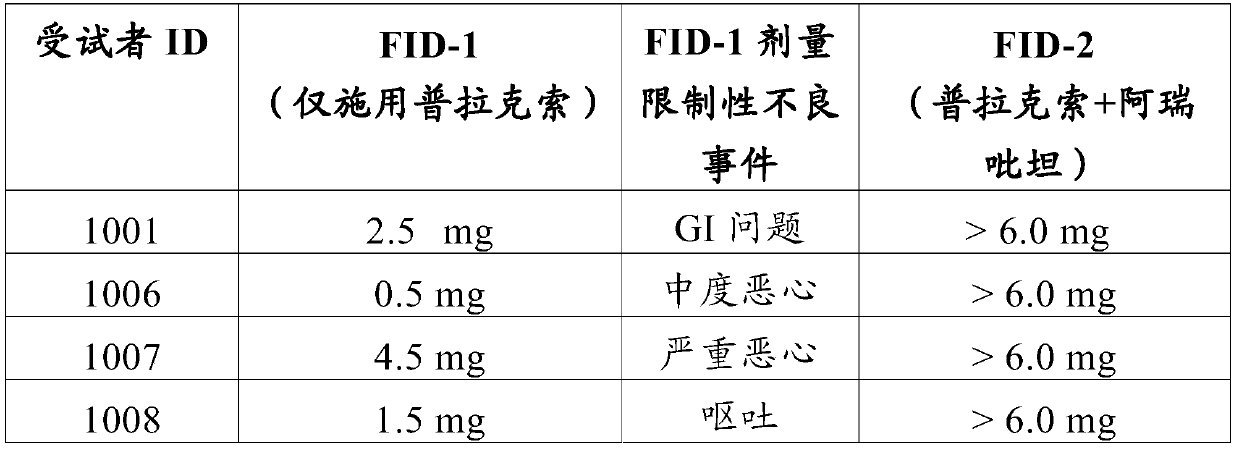
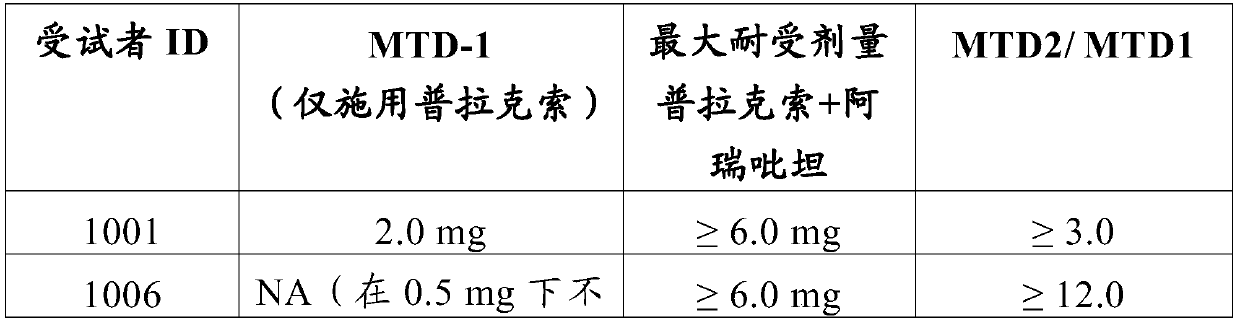
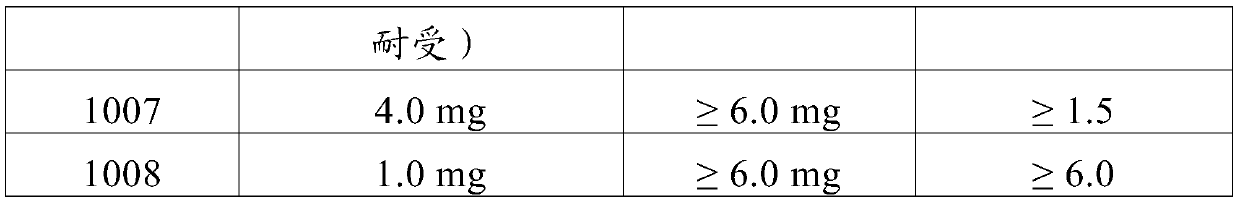
[0279] The ability of NK1-antagonists to prevent the adverse effects of 6-propylamino-4,5,6,7-tetrahydro-1,3-benzothiazol-2-amine in humans was tested.
[0280] A Phase I study was conducted in subjects receiving oral doses of pramipexole dihydrochloride monohydrate ("pramipexole") with or without aprepitant. The study was a single-center, single-blind, placebo-controlled study.
[0281] The purpose of this study was to demonstrate that aprepitant can safely alleviate the gastrointestinal side effects of pramipexole at therapeutic and even supratherapeutic doses.
[0282] To enroll in the study, participants (ages 18 to 60) must be in good health during the study period, refrain from drinking beverages containing xanthines, quinine and caffeine, and refrain from prolonged periods of intense physical activity. All subjects signed an informed consent form, indicating that they understood the purpose of the study and the procedures required for the study, and were willing to par...
Embodiment 2
[0299] A Phase I study was conducted in subjects who received a single oral dose of pramipexole dihydrochloride monohydrate ("pramipexole") with or without a single oral dose of aprepitant. The study was a single-center, single-blind study.
[0300] The purpose of this study was to demonstrate that aprepitant can safely relieve pramipexole given at doses equal to or higher than those approved in the treatment of Parkinson's disease or shown to be effective in the treatment of depression in clinical trials gastrointestinal side effects.
[0301] To enroll in the study, participants must meet the following key inclusion / exclusion criteria:
[0302] key inclusion criteria
[0303] 1. Include male and female subjects aged 20-45 years (inclusive).
[0304] 2. Females of childbearing potential must agree to abstain from alcohol or otherwise use the following two medically acceptable forms of contraception from the screening period until 14 days after the study withdrawal visit: c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com