Processes of making l-ornithine phenylacetate
A technology of ornithine hydrochloride and phenylacetate, applied in the fields of medicinal chemistry, biochemistry and medicine, can solve the problems of danger, difficult intravenous administration, and undesired
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
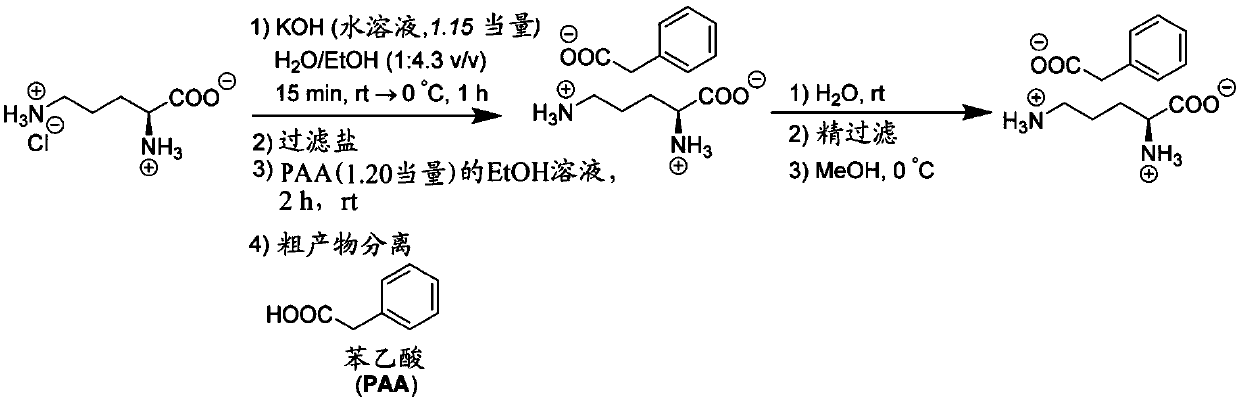
[0062] Example 1: Large scale batch process for the preparation of crude L-ornithine phenylacetate
[0063] Charge 4.05kg (61.38mol) KOH in the first reactor, dissolve it in 10.4L (10.4kg) H 2 O and stirred until a clear solution formed. Subsequently, 9.00 kg (53.37 mol) of L-ornithine hydrochloride was added to the KOH solution in two parts at about 15-25° C. to form a suspension. Subsequently, 45.0 L (35.5 kg) of ethanol was added to the suspension at 15-25 °C and stirred for about 15-20 min. The suspension is then cooled to about 0-5°C and stirred at this temperature for at least 60 min, but not more than 90 min. Separately, 8.72kg (64.05mol) of phenylacetic acid (PAA) was dissolved in 36.0L (28.4kg) of ethanol in a second reactor and stirred at 15-25°C until completely dissolved. The cold suspension from the first reactor was filtered by depth filtration into the PAA solution to remove precipitated KCl, and the filter cake was washed with about 18.0 L (14.2 kg) of eth...
Embodiment 2
[0065] Example 2: Improved large-scale batch process for the preparation of crude L-ornithine phenylacetate
[0066] Charge 4.05kg (61.38mol) KOH in the first reactor (100L), dissolve it in 10.4L (10.4kg) H 2 O and stirred until a clear solution formed. Subsequently, 9.00 kg (53.37 mol) of L-ornithine hydrochloride was added to the KOH solution in two parts at about 15-25° C. to form a suspension. Subsequently, 45.0 L (35.5 kg) of ethanol was added to the suspension at 15-25° C., and stirred for about 15-20 min. The suspension is then cooled to about 0-5°C and stirred at this temperature for at least 60 min, but not more than 90 min. Separately, 8.72kg (64.05mol) of phenylacetic acid (PAA) was dissolved in 36.0L (28.4kg) of ethanol in a second reactor (450L) and stirred at 15-25°C until completely dissolved. The cold suspension from the first reactor was filtered by depth filtration into the PAA solution to remove precipitated KCl, and the filter cake was washed with abou...
Embodiment 3
[0069] Example 3: Recrystallization of L-ornithine phenylacetate
[0070] In the first container, add the crude product L-ornithine phenylacetate of 13.12kg (48.89mol) embodiment 2, then add 32.8L (32.8kg) H 2 O, and stirred at 15-25°C for about 15-30min until completely dissolved. The resulting solution was then filtered through a particle filter (0.2 μm) into a second container. The particle filter was washed with 262.4 L (207.8 kg) methanol into a second vessel and a suspension formed. The suspension was cooled to 0-5°C and stirred at 0-5°C for about 60 min, but not more than 90 min. A crystalline solid (L-ornithine phenylacetate) precipitated after cooling. The precipitate was separated by centrifugation and washed with 52.5 L (42.6 kg) of methanol. The final product was vacuum dried at about 50 °C for at least 10 h. The dried product was delumped by grinding (1.0 mm sieve). Yield: Overall 70.5% (9.72 kg) (74.1% yield from recrystallization only). The recrystalliz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com