Formulations of L-ornithine phenylacetate
A technology of phenylacetate and ornithine, which is applied in the field of L-ornithine phenylacetate preparations, can solve problems such as exhaustion and sudden deterioration of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1——preparation of coating controlled release preparation
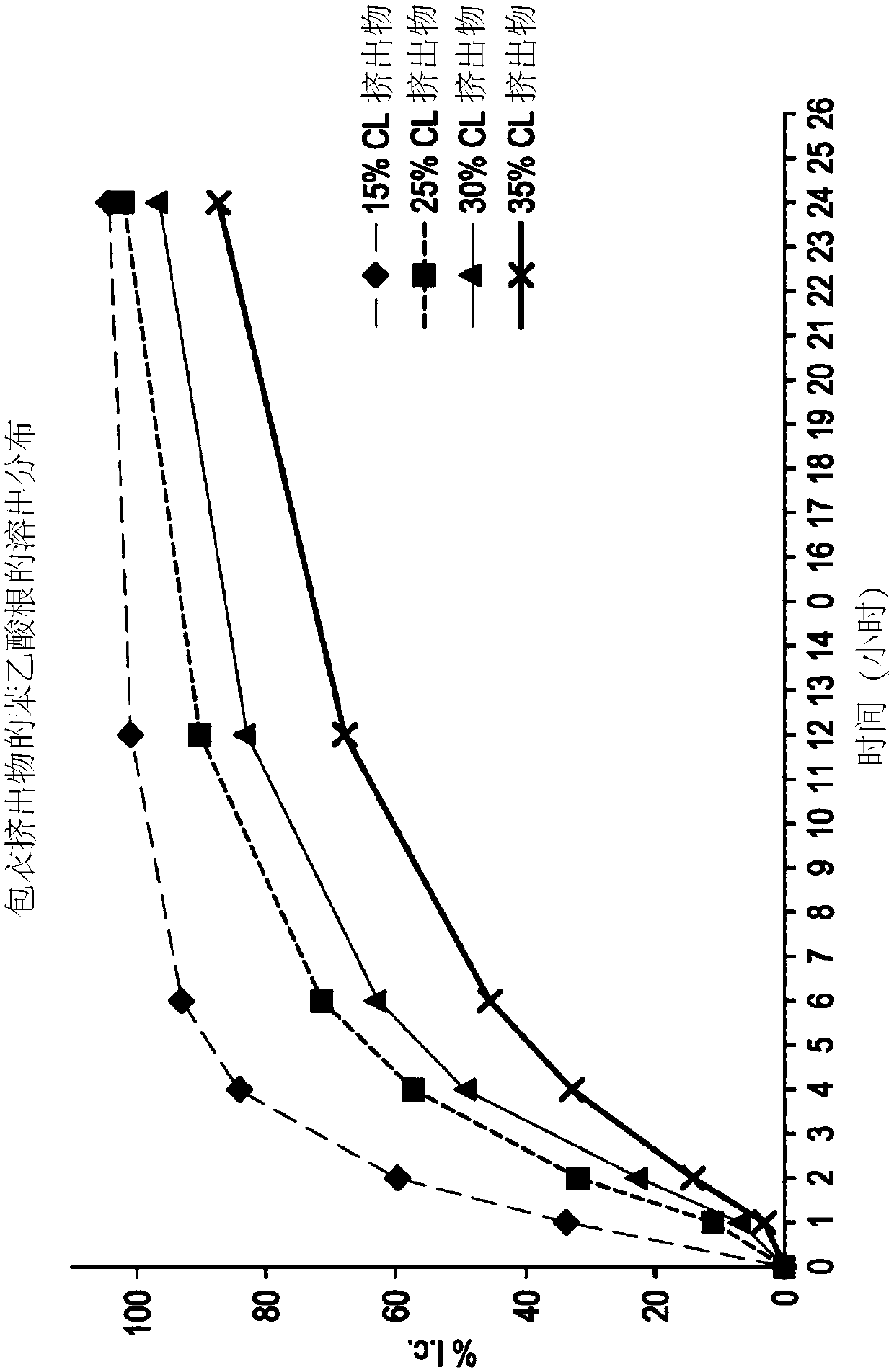
[0094] Three controlled release oral pharmaceutical formulations of L-ornithine phenylacetate (Formulations 3, 5 and 6) were prepared. Formulations 3 and 6 contained sugar spheres ("Suglets") onto which a mixture of L-ornithine phenylacetate and hydroxypropylmethylcellulose (HPMC) in aqueous solution was sprayed and layered to an approximate weight percent of 35%. After drying, use ethyl cellulose ("ETHOCEL TM ”) solutions in organic solvents to further coat these spray-layered beads or pellets. By adjusting the amount of ethylcellulose coating applied, the release profile of the pellets can be tailored to produce the desired Release profile. This profile can be measured in vitro, e.g. by dissolution results, or by in vivo techniques, where the pharmacokinetic distribution of the drug in plasma (or other biological location, such as urine) is assessed. Formulation 3 describes For 25% coated beads...
Embodiment 2
[0096] Example 2 - Pharmacokinetic studies in dogs
[0097] A pharmacokinetic study of a controlled-release oral formulation of L-ornithine phenylacetate was performed in dogs. Two groups (three each) of 6 dogs were used in the study. Controlled release formulations (formulations 3, 5 and 6 described in Example 1) were administered orally at a dose of 200 mg / kg. An immediate release formulation of L-ornithine phenylacetate in water was administered orally at a dose of 50 mg / kg. The AUC and plasma Cmax for the analyte L-ornithine and the phenylacetate metabolites phenylacetylglutamine (PAGN) and phenylacetic acid (PAA) are shown in image 3 middle. All controlled release formulations produced comparable PAGN and ORN pharmacokinetics, and the controlled release formulations had equal plasma Cmax to AUC ratios for PAA and PAGN.
[0098] The in vivo PK data of the metabolite PAA and the in vivo surrogate PD data of the metabolite PAGN in animal models were collected and show...
Embodiment 3
[0099] Example 3 - Preparation of Large Pellets of Coated Controlled Release Formulation
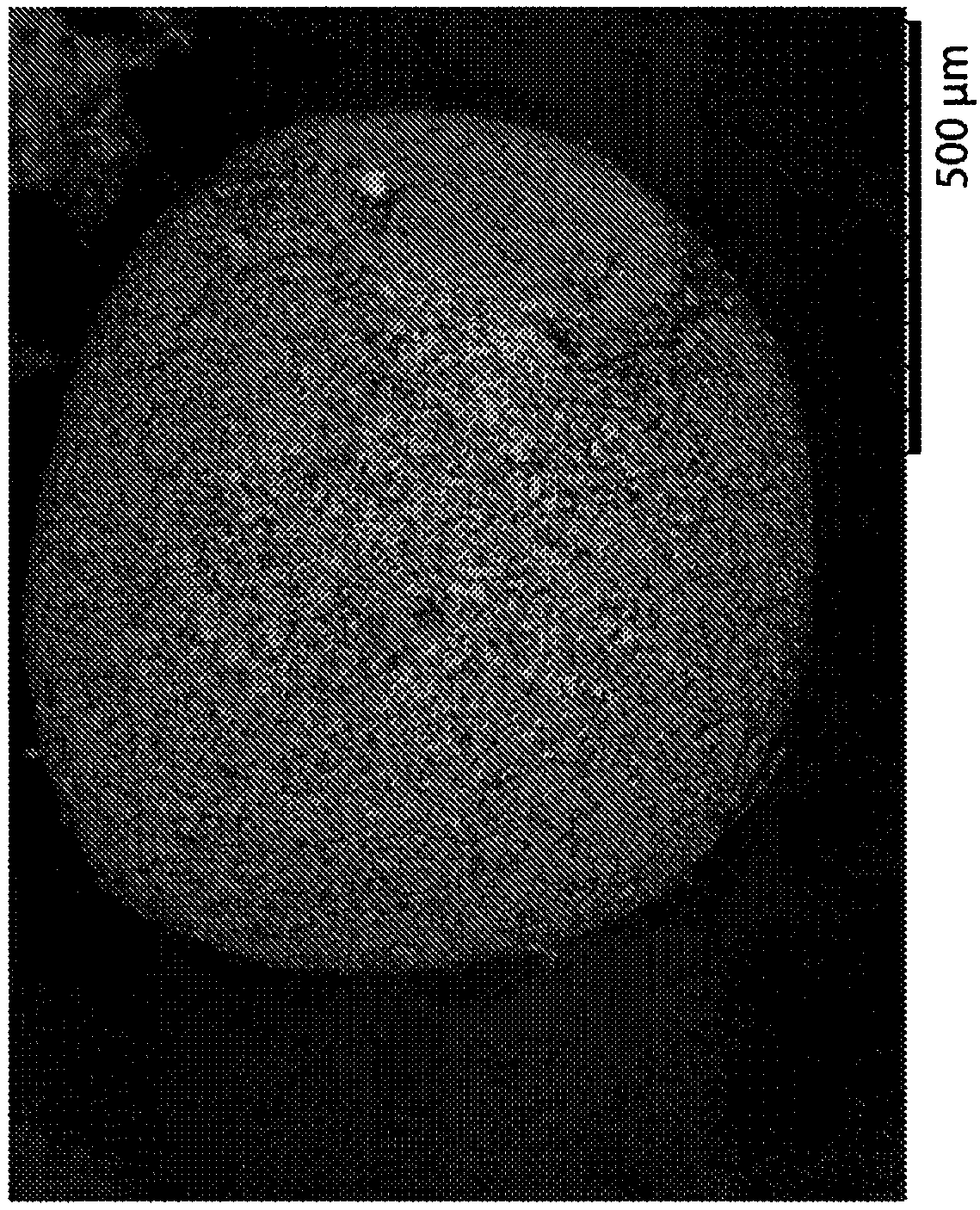
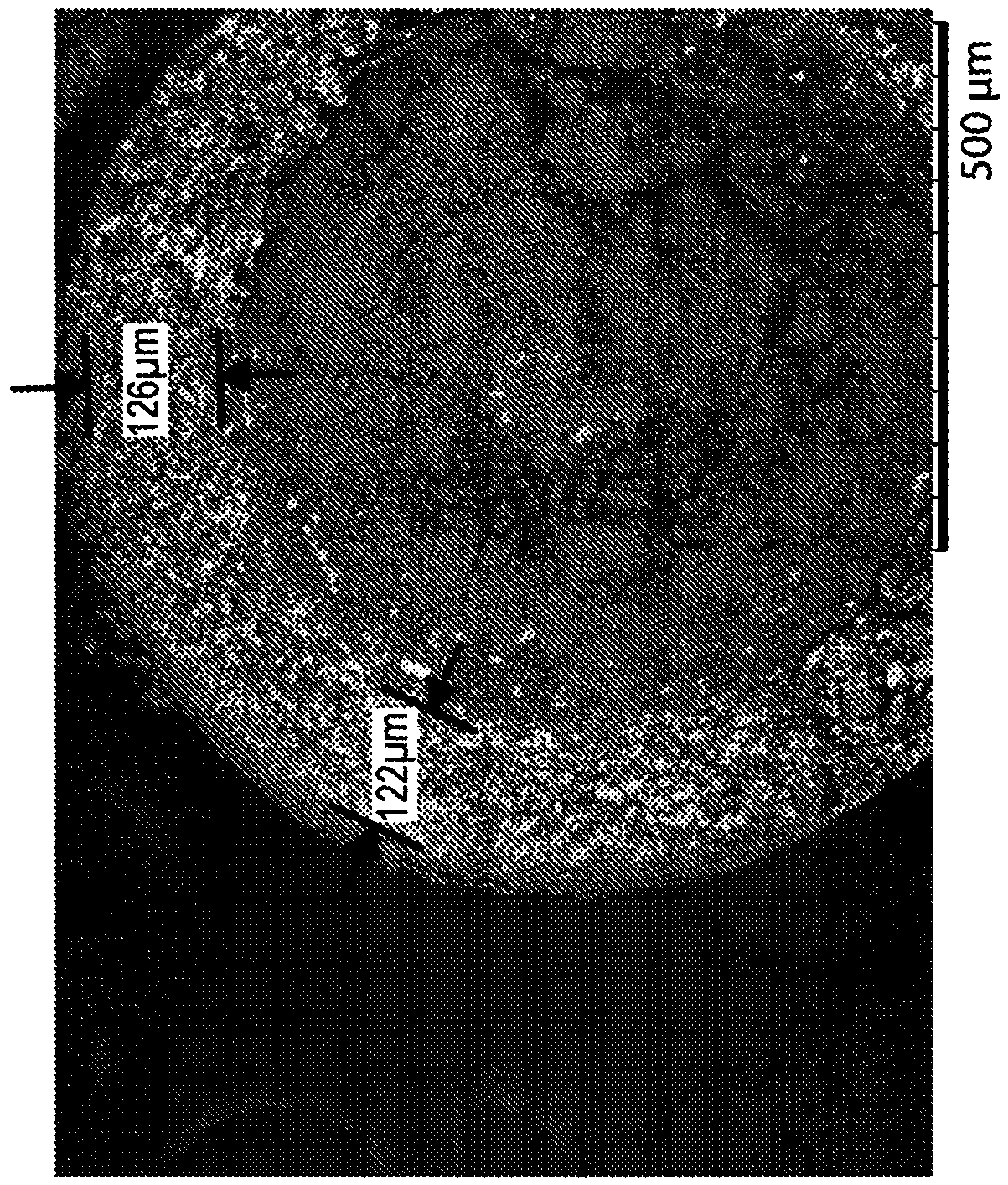
[0100] Three controlled release oral pharmaceutical formulations of L-ornithine phenylacetate (Formulations A, B and C) were prepared following similar procedures as described in Example 1. Sugar spheres with an average diameter between 500 and 600 μm were used as cores. A mixture of L-ornithine phenylacetate and hydroxypropylmethylcellulose (HPMC) in water was then sprayed and layered onto the sugar spheres. In each case, the excipient talc was also used in forming the layered core pellets.
[0101] After drying, these layered core pellets were further coated with a slow release polymer selected from ethylcellulose or Eudragit NM 30 D. The components of Formulations A, B and C are summarized in Table 1 below. The final pellets in Formulations A, B and C contained 15%, 30% or about 41% ER coating relative to the total weight of the pellets, respectively. The average diameter of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com