Cathepsin inhibitor as well as preparation method and application thereof
A C1-C6, alkyl technology, applied in cathepsin inhibitors and their preparation, the application field in the preparation of antitumor drugs, can solve the problems of multi-drug resistance, difference in apoptosis sensitivity, affecting the effect of chemotherapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
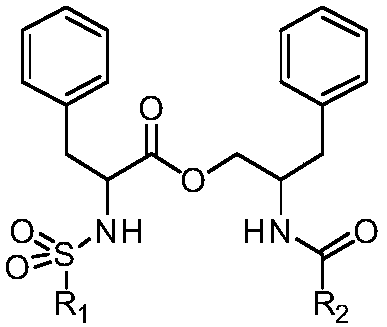
[0189] Example 1: General method for the preparation of (R)-N-substituted phenylsulfonyl phenylalanine-(S or R)-2-substituted benzamido-3-phenylpropyl ester (1)
[0190] Step A: General method for the preparation of (R or S)-N-tert-butoxycarbonylphenylalanine-(S or R)-2-substituted benzamido-3-phenylpropyl ester
[0191] N-tert-butoxycarbonyl (Boc)-D(or L)-phenylalanine (0.2g, 0.75mmol) was dissolved in dry dichloromethane (10ml) and (N,N')-carbonyldiimidazole was added (0.15g, 0.93mmol), stirred at room temperature for 30min. Then various N-(substituted benzoylated)-phenylalaninols (0.28 g, 0.75 mmol) diluted with dichloromethane (10 ml) were added into the reaction system, and finally reacted overnight at room temperature. Silica gel column chromatography (chloroform:acetone=500:1) gave a white solid with a yield of 64-85%.
[0192] Step B:
[0193] General method for the preparation of (R or S)-N-substituted phenylsulfonyl phenylalanine-(S or R)-2-substituted benzamido-3...
Embodiment 2
[0195] Example 2: Preparation of (R)-N-(4-bromobenzenesulfonyl)-phenylalanine-(S)-2-benzamido-3-phenylpropyl ester
[0196] The synthetic method is the same as that of Example 1, and the productive rate is 89%. 1 H-NMR (600MHz, CDCl 3 ): δ7.00-7.75(m, 19H, ArH), 6.53(d, 1H, J=7.8Hz, 6-NH), 5.18(d, J=9.0Hz, 1H, 7-NH), 4.61(m ,1H,4-H),4.34-4.36(d,J=9.6Hz,1H,3a-H),4.11(d,J=5.4Hz,1H,2-H),4.03(dd,J=7.2Hz ,1H,3b-H),3.06-3.10(dd,J=13.8,5.4Hz,1H,1a-H),2.97-3.00(dd,J=13.8,6.6Hz,1H,5a-H),2.87- 2.93 (dd, J=13.8, 6.6Hz, 2H, 1b-H, 5b-H); 13 C-NMR (150MHz, CDCl 3 ):δ170.6,167.1,138.2,136.9,134.8,134.0,132.2×2,131.6,129.2×2,129.0×2,128.7×2,128.7×2,128.4×2,128.4×2,127.8,127.3,127.0×2,126.8,66.2,57.3,49.8,38.7,37.2.
Embodiment 3
[0197] Example 3: Preparation of (R)-N-(4-bromobenzenesulfonyl)-phenylalanine-(R)-2-benzamido-3-phenylpropyl ester
[0198] The synthetic method is the same as in Example 1, and the productive rate is 85%. 1 H-NMR (600MHz, CDCl 3 ):δ7.02-7.73(m,19H,ArH),6.45(d,1H,J=8.4Hz,6-NH),5.17(d,J=9.6Hz,1H,7-NH),4.59(m ,1H,4-H),4.25-4.28(dd,J=11.4,3.0Hz,1H,3a-H),4.17-4.21(dd,J=14.4,8.4Hz,1H,2-H),4.12- 4.15(dd, J=11.4, 4.8Hz, 1H, 3b-H), 3.08-3.11(dd, J=13.8, 5.4Hz, 1H, 1a-H), 2.98-2.95(dd, J=13.8, 6.6Hz ,1H,5a-H),2.92-2.88(dd,J=13.8,6.6Hz,1H,1b-H),2.83-2.87(dd,J=13.8,6.6Hz,1H,5b-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com