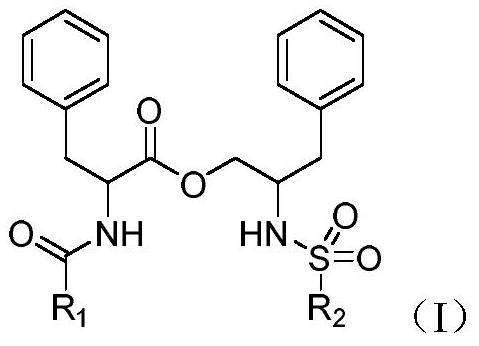
Cathepsin inhibitor and its preparation method and application
A cathepsin and composition technology, applied in the preparation of sulfonic acid amide, drug combination, organic chemistry and other directions, can solve the problems of multi-drug resistance, difference in apoptosis sensitivity, affecting the effect of chemotherapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
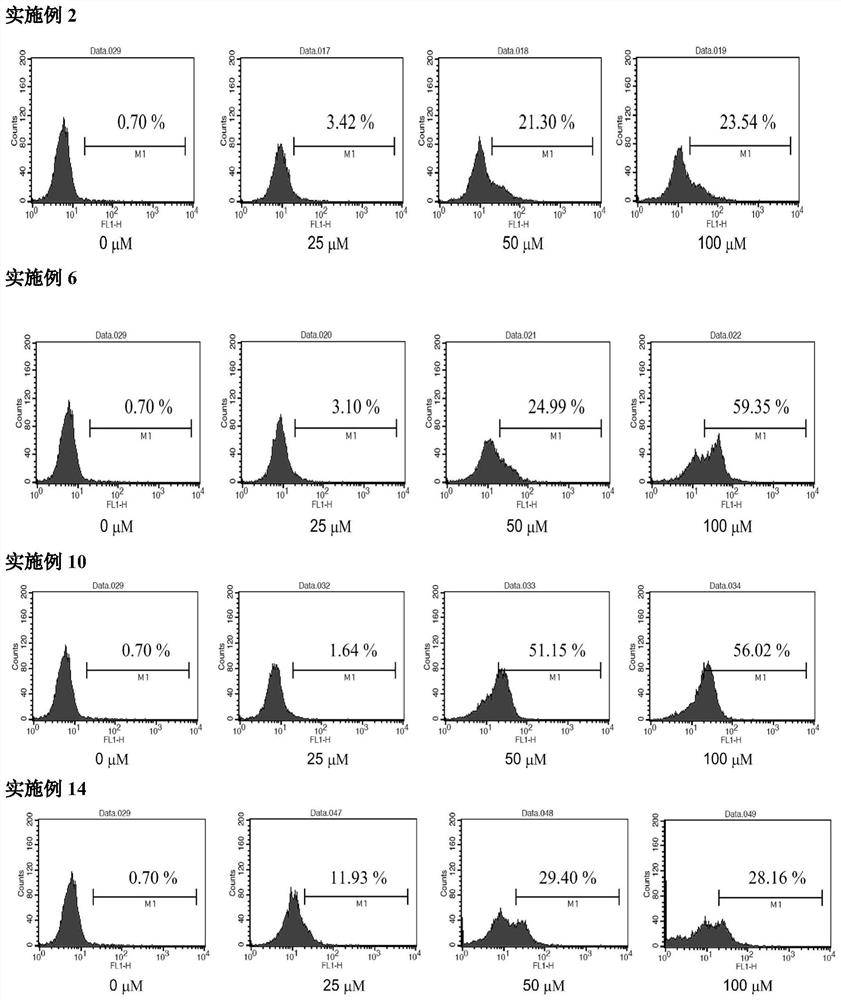
Examples
Embodiment 1
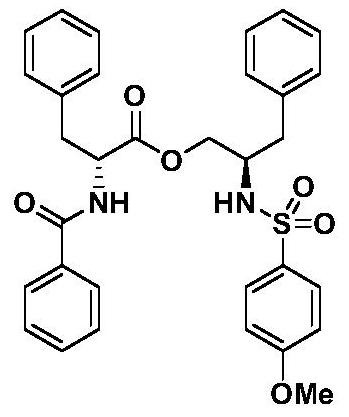
[0112] Embodiment 1: General method for the preparation of (R)-N-benzoylphenylalanine-(S)-2-(4-methoxyl-benzenesulfonamido)-3-phenylpropyl ester
[0113] Step A:
[0114] Dissolve L-phenylalaninol (0.5g, 3.31mmol), triethylamine (0.92ml, 6.62mmol) in dichloromethane (35ml) and cool to 0°C, slowly dissolve 4-methoxy-benzenesulfonyl chloride (0.85g, 3.33mmol) was added dropwise to the reaction liquid, and reacted overnight at room temperature under the protection of nitrogen. Quench the reaction with 5% sodium bicarbonate solution (4ml) and 1M hydrochloric acid solution (7ml), separate the layers, extract the aqueous phase with dichloromethane (2×6ml), combine the organic phases, wash with saturated brine (3× 10ml) was washed, dried over anhydrous sodium sulfate, and subjected to silica gel column chromatography (chloroform:acetone=300:1) to obtain 1.09g of white solid (1), with a yield of 97%.
[0115] Step B:
[0116] General method for the preparation of (R)-N-tert-butoxyc...
Embodiment 2
[0121] Example 2: Preparation of (R)-N-benzoylphenylalanine-(R)-2-(4-methoxy-benzenesulfonamido)-3-phenylpropyl ester
[0122] The synthetic method is the same as that of Example 1, and the productive rate is 92%. 1 H-NMR (600MHz, CDCl 3 ): δ6.83-7.75 (m, 19H, ArH), 6.75 (d, 1H, J=7.8Hz, 6-NH), 5.19 (d, J=7.8Hz, 1H, 7-NH), 4.95-4.99 (dd, J=13.8, 7.2Hz, 1H, 2-H), 4.16-4.19 (dd, J=10.4, 4.2Hz, 1H, 3a-H), 3.87-3.90 (dd, J=10.4, 4.2Hz, 1H, 3b-H), 3.81(s, 3H, 8-H), 3.62(m, 1H, 4-H), 3.23-3.27(dd, J=13.8, 6.6Hz, 1H, 1a-H), 3.17 -3.21, (dd, J=13.8, 6.6Hz, 1H, 1b-H), 2.67-2.70 (dd, J=13.8, 7.8Hz, 1H, 5a-H), 2.62-2.65 (dd, J=13.8, 6.6Hz, 1H, 5b-H).
Embodiment 3
[0123] Example 3: Preparation of (S)-N-benzoylphenylalanine-(R)-2-(4-methoxy-benzenesulfonamido)-3-phenylpropyl ester
[0124] The synthetic method is the same as that of Example 1, and the productive rate is 92%. 1 H-NMR (600MHz, CDCl 3 ): δ6.86-7.76 (m, 19H, ArH), 6.70 (d, 1H, J=9.0Hz, 6-NH), 4.97-5.01 (dd, J=13.8, 6.6Hz, 1H, 2-H) , 4.80(d, J=8.4Hz, 1H, 7-NH), 4.05-4.06(dd, J=4.8Hz, 2H, 3-H), 3.85(s, 3H, 8-H), 3.60(m, 1H, 4-H), 3.18-3.26 (m, J=2H, 1-H), 2.61-2.63 (dd, 2H, 5-H); 13 C-NMR (150MHz, CDCl 3 ): δ171.4, 167.1, 162.7, 136.1, 135.9, 133.5, 131.9, 131.7, 130.1, 129.2×2, 129.1×2, 129.0×2, 128.8×2, 128.7×2, 128.6×2, 127.2, 127.0, 125.8, 114.2×2, 65.9, 55.5, 53.8, 53.6, 38.2, 37.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com