Preparation method of recombinant human C-reactive protein
A reactive protein and reaction technology, applied in the field of preparation of recombinant human C-reactive protein, can solve the problems of difficulty in renaturation, inability to use clinical detection, inability to perform ELISA detection, etc., and achieve the beneficial effects of improving yield and purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
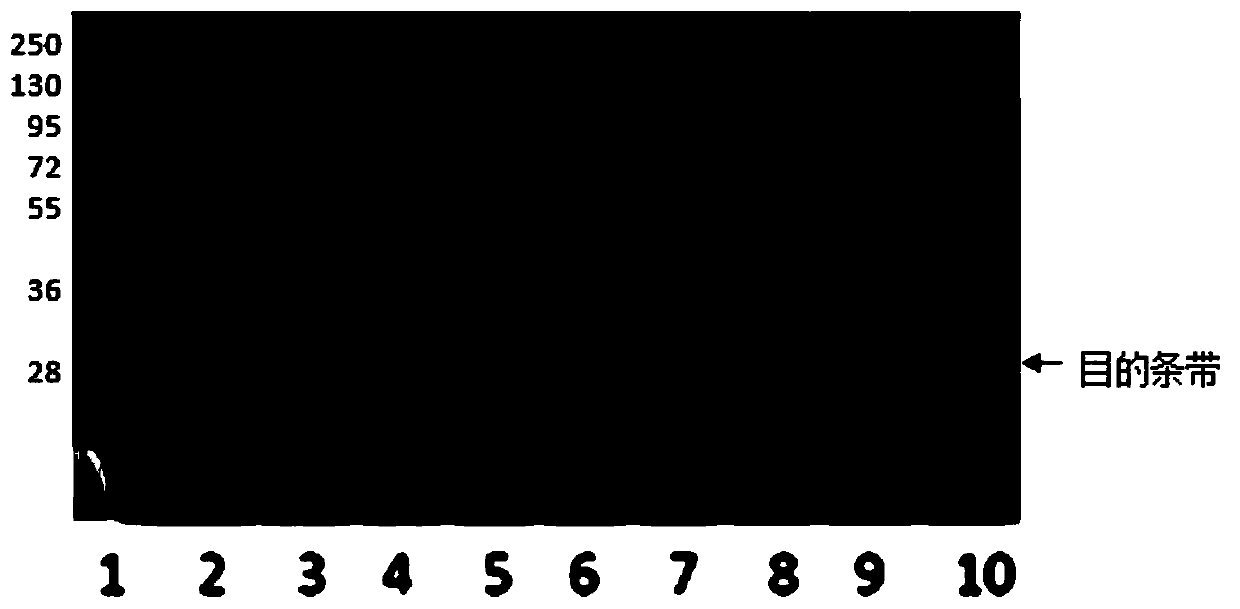
[0031] The prokaryotic expression and purification process of recombinant human C-reactive protein is as follows:
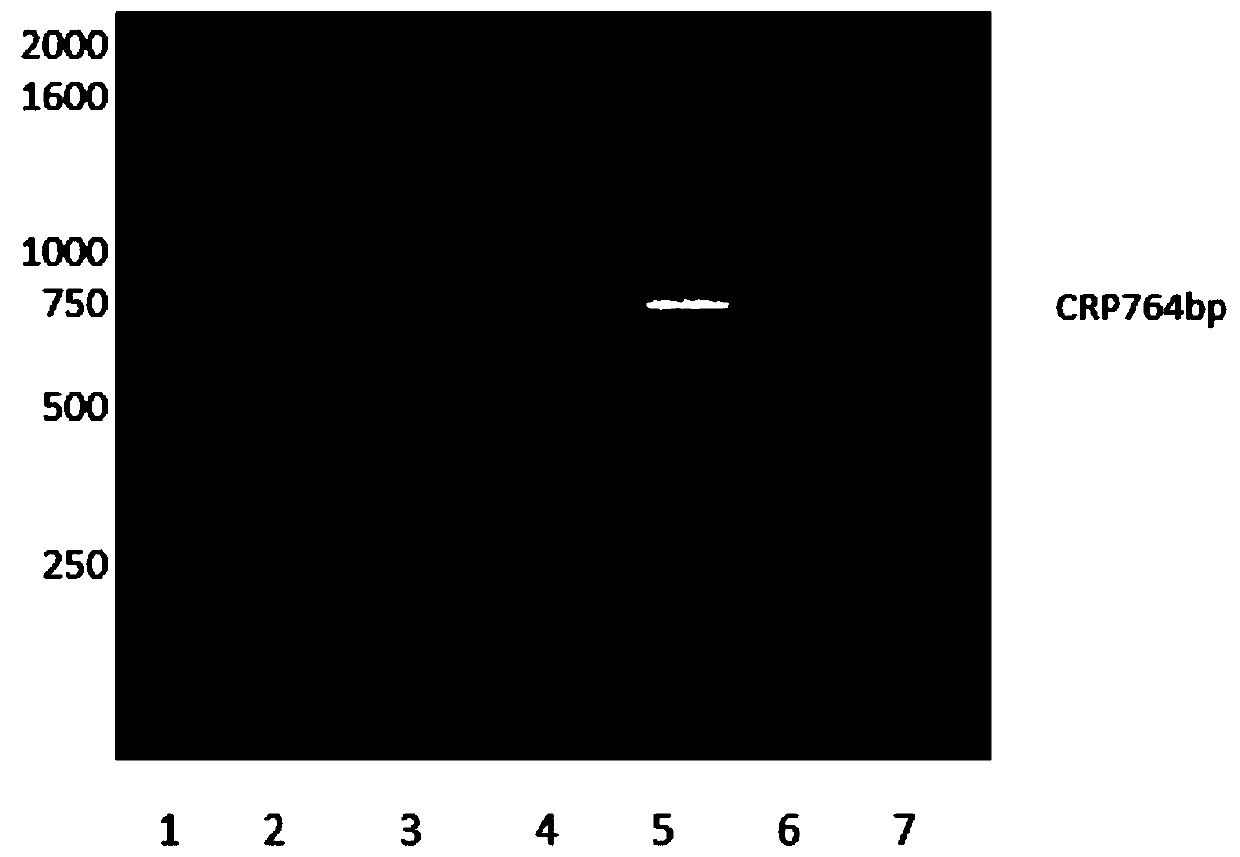
[0032] (1) Cloning of human C-reactive protein gene
[0033] The amino acid sequence of human C-reactive protein was obtained from NCBI, and the C-reactive protein coding gene was artificially optimized and synthesized according to the codon preference characteristics of E. (-10×His-) is used as a purification tag. The amino acid sequence of the specific protein is shown in SEQ ID NO: 1, and the nucleotide sequence of the protein-coding gene is shown in SEQ ID NO: 2.
[0034] Design primers for PCR reaction to amplify the synthetic gene, and introduce Nde I and Xho I restriction sites at both ends of the primers. See SEQ ID NO: 3 for the sequence of the forward primer and SEQ ID NO: for the sequence of the reverse primer. 4. The PCR conditions are as follows:
[0035] Pre-denaturation at 94°C for 5 minutes,
[0036]
[0037] Extension at 72°C for 10 min te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com