Pretreatment method, pretreatment solution, kit and use thereof for viral nucleic acid detection
A technology of pretreatment solution and viral nucleic acid, which is applied in microorganism-based methods, biochemical equipment and methods, and microbial determination/inspection, etc. Conducive to long-term storage and detection, improving detection efficiency and shortening detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
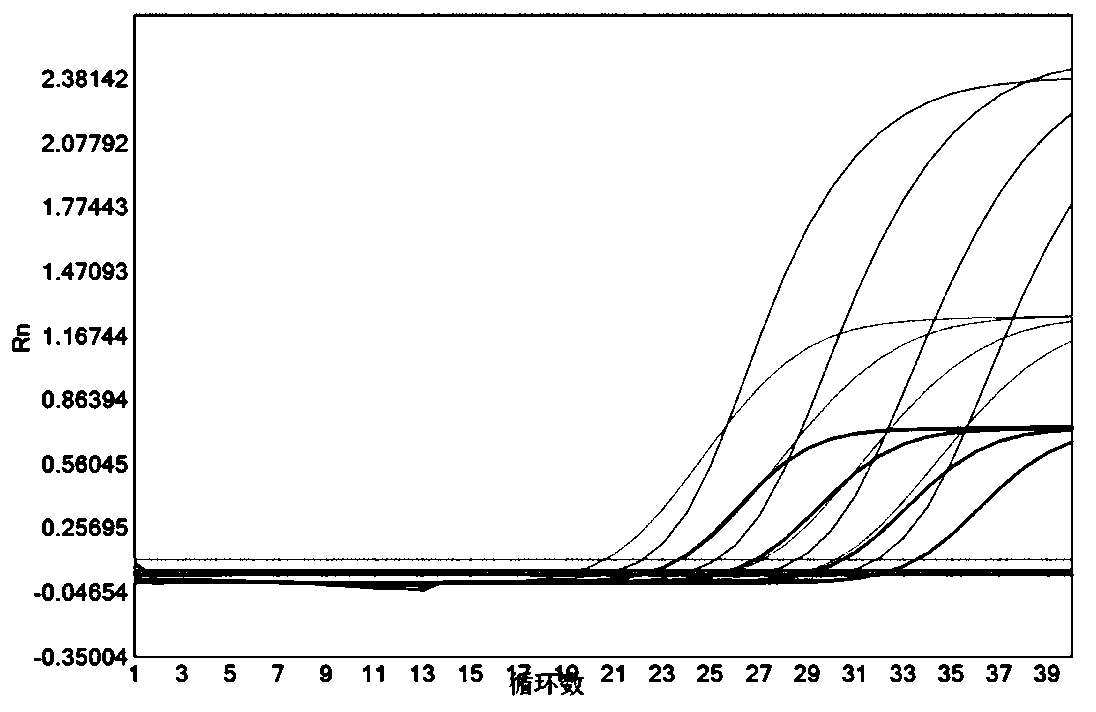
[0053] Example 1 The present invention is used for the pretreatment and rapid detection of respiratory syncytial virus (RSV) oropharyngeal swab samples
[0054] In order to evaluate the virus pretreatment solution in this invention, the virus pretreatment solution of this invention (the concentration of Tris-HCl is 100mM, the concentration of EDTA-2Na is 10mM, the concentration of sodium chloride is 0.9% (w / v), The concentration of RNasin was 20U / mL, and the concentration of Proclin 950 was 0.04% (v / v)) were compared with normal saline and commercial virus pretreatment solution. The method for comparison is pretreatment with a clinically diagnosed positive respiratory syncytial virus (RSV) throat swab sample diluted (1:9, v / v). hours / 24 hours / 48 hours and 72 hours for direct amplification of samples, and by comparing the detection efficiency of real-time fluorescent quantitative PCR (real-timeqPCR) under room temperature pretreatment conditions by Ct value, to evaluate the eff...
Embodiment 2
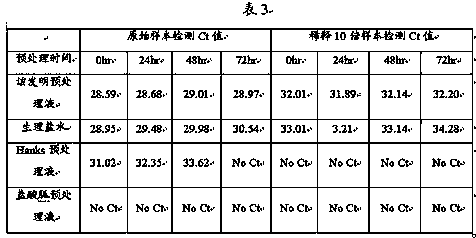
[0059] Example 2 The present invention is used for nucleic acid pretreatment and rapid detection after purification of 2019 novel coronavirus (2019-nCoV) samples
[0060] In order to evaluate the virus pretreatment solution in this invention, the virus pretreatment solution of this invention (the concentration of Tris-HCl is 100mM, the concentration of EDTA-2Na is 10mM, the concentration of sodium chloride is 0.9% (w / v), The concentration of RNasin was 20U / mL, and the concentration of Proclin 300 was 0.01% (v / v)) were compared with normal saline and commercial virus pretreatment solution. The method for comparison is to dilute (1:9, v / v) pretreatment with the nucleic acid of the 2019 novel coronavirus (2019-nCoV) that is clinically diagnosed as positive. hours / 24 hours / 48 hours and 72 hours for direct amplification of samples, and by comparing the detection efficiency of real-time fluorescent quantitative PCR (real-timeqPCR) under room temperature pretreatment conditions by Ct...
Embodiment 3
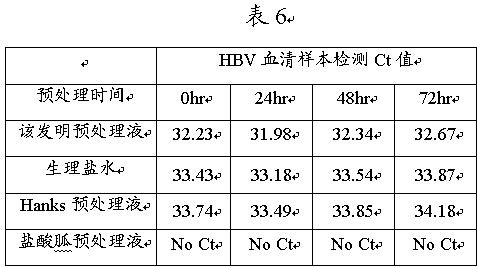
[0063] Example 3 The present invention is used for pretreatment and rapid detection of enterovirus versatility (EV) throat swab samples
[0064] In order to evaluate the virus pretreatment solution in this invention, the virus pretreatment solution of this invention (the concentration of Tris-HCl is 100mM, the concentration of EDTA-2Na is 10mM, the concentration of sodium chloride is 0.9% (w / v), The concentration of SDS was 0.1%, and the concentration of Proclin950 was 0.04% (v / v)) were compared with normal saline and commercial virus pretreatment solution. The method for comparison is pretreatment with the common enterovirus (EV) throat swab samples clinically diagnosed as positive (1:9, v / v). The pretreatment condition is room temperature 25°C. Samples were directly amplified at 0 hours / 24 hours / 48 hours and 72 hours, and the detection efficiency of real-time qPCR (real-time qPCR) under room temperature pretreatment conditions was compared by Ct value to evaluate the effect ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com