A kind of peptide map analysis method of nesiritide and its application
An analytical method, nesiritide technology, applied in analytical materials, material separation, instruments, etc., can solve problems such as early peaks, time-consuming, analytical deviations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
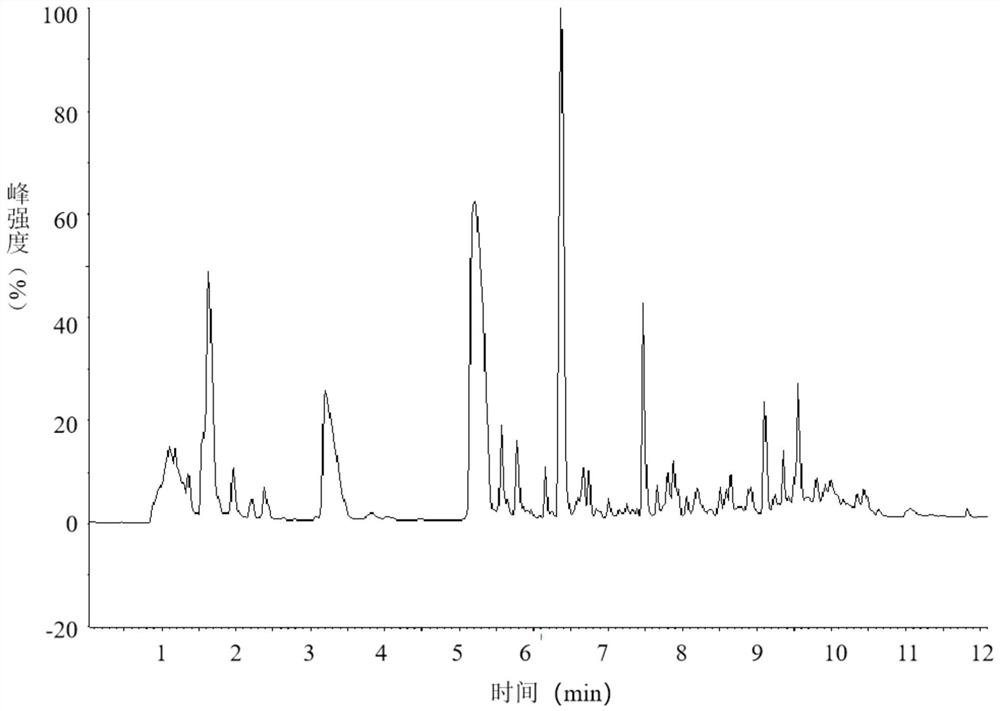
[0056] This embodiment provides a method for analyzing peptide maps of nesiritide, which specifically includes the following steps:
[0057] (1) Reduction and alkylation of nesiritide samples
[0058] Dilute the sample with water to 1.5mg / mL, add it to a 1.5mL centrifuge tube, add 100μL 0.1mol / L ammonium bicarbonate buffer, then add 11.56μL 0.1mol / L DTT (dithiothreitol), mix well, and add Incubate at 56 °C for 1 h, and after incubation, place it in a 20 °C water bath to cool for 1 min.
[0059] Add 12.6 μL of 0.25 mol / L iodoacetamide solution to the reduced sample, mix well, and incubate at 20°C for 1 h in the dark.
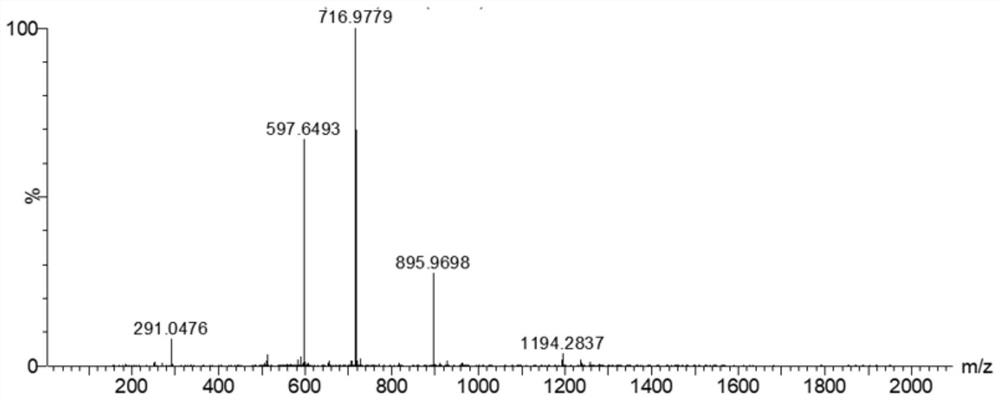
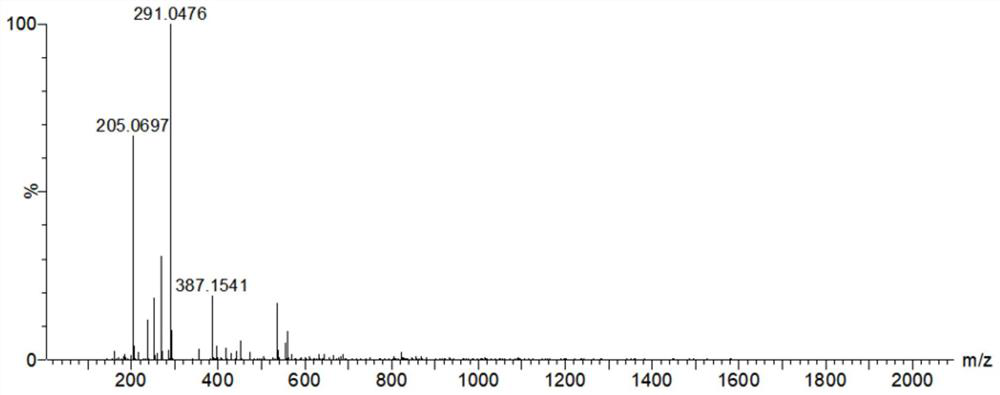
[0060] After the reaction, use mass spectrometry to confirm that the reduction and alkylation are complete (e.g. figure 1 shown), four mass spectral peaks can be observed in the figure, with figure 2 The comparison of the mass spectra of the unreduced and alkylated nesiritide shows that the reduction and alkylation are complete, and the disulfide bond of nesi...
Embodiment 2
[0074] This embodiment provides a method for analyzing peptide maps of nesiritide, which specifically includes the following steps:
[0075] (1) Reduction and alkylation of nesiritide samples
[0076] Dilute the sample with water to 1.5mg / mL, add it to a 1.5mL centrifuge tube, add 100μL 0.4mol / L ammonium bicarbonate buffer, then add 11.56μL 0.4mol / L DTT (dithiothreitol), mix well, and add Incubate at 56 °C for 1 h, and after incubation, place it in a 25 °C water bath to cool for 1 min.
[0077] Add 12.6 μL of 0.5 mol / L iodoacetamide solution to the reduced sample, mix well, and incubate at 25°C for 1 h in the dark.
[0078] After the reaction, use MS detection to confirm that the reduction and alkylation are complete (such as Figure 4 shown), four mass spectral peaks can be observed in the figure, with figure 2 The comparison of the mass spectra of unreduced and alkylated in 2000 shows that the reduction and alkylation are complete, and the disulfide bond of nesiritide is c...
Embodiment 3
[0089] This embodiment provides a method for analyzing peptide maps of nesiritide, which specifically includes the following steps:
[0090] (1) Reduction and alkylation of nesiritide samples
[0091] Dilute the sample with water to 1.5mg / mL, add it to a 1.5mL centrifuge tube, add 100μL 0.2mol / L ammonium bicarbonate buffer, then add 11.56μL 0.2mol / L DTT, mix well, and incubate at 56°C for 1h, incubate After that, it was cooled in a 15°C water bath for 1 min.
[0092] Add 12.6 μL of 0.5 mol / L iodoacetamide solution to the reduced sample, mix well, and incubate at 15°C for 1 h in the dark.
[0093] After the reaction, use MS detection to confirm that the reduction and alkylation are complete (such as Figure 4 shown), four mass spectral peaks can be observed in the figure, with figure 2 The comparison of the mass spectra of unreduced and alkylated in 2000 shows that the reduction and alkylation are complete, and the disulfide bond of nesiritide is completely opened.
[0094...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com