Novel compounds
A compound, pharmaceutical technology, applied in the field of novel compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] Preparation of compounds of formula (Ia)
[0074]
[0075] Compounds of formula (Ia) are alkoxyspiro compounds of formula (I), which can be deprotected according to Scheme 1 (below) by deprotecting the Boc protecting group of alkoxyspiro compounds of formula (II) with, for example, TFA And further prepared by coupling the amino group of TFA salt with 4,4,4-trifluorobutyric acid. The intermediate compound of formula (II) can be prepared by reacting the corresponding commercially available alcohol (ROH) with a halospiro N-Boc protected compound of formula (IV), the synthesis of which is described below.
[0076]
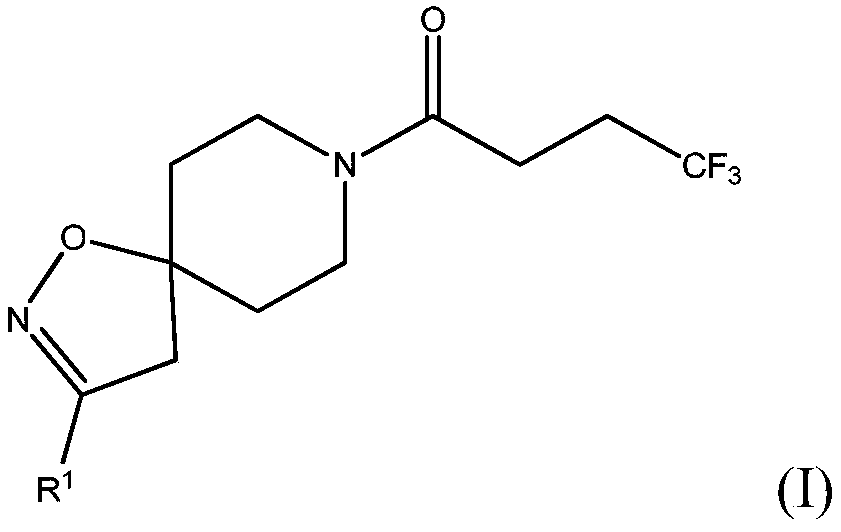
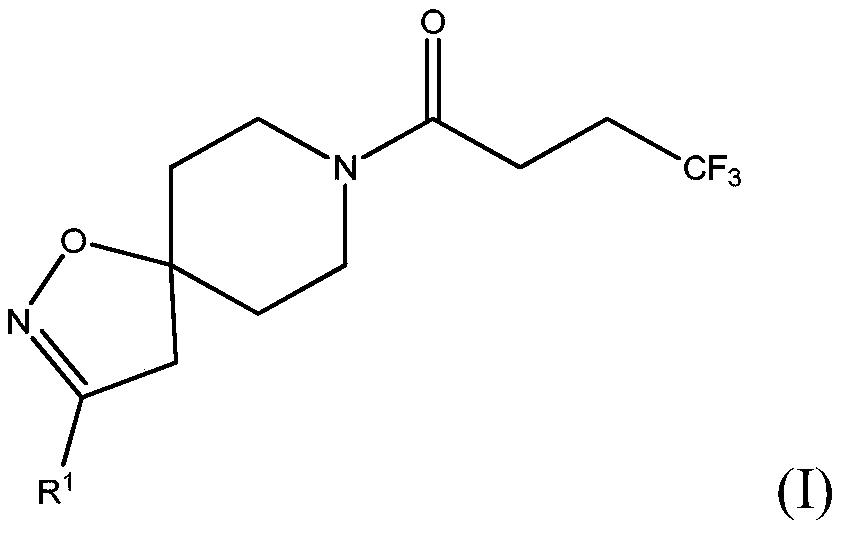
[0077] Scheme 1 - Preparation of compounds of formula (I), wherein R 1 for C 1-6 Straight-chain alkoxy; C 3-4 Branched alkoxy; methoxy substituted with one or more fluorines; or ethoxy substituted with one or more fluorines
[0078]Alternatively, it can be prepared by reacting the corresponding commercially available alcohol (ROH) with a halospirocycli...
Embodiment 1
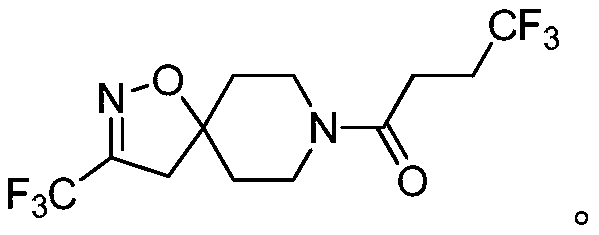
[0236] Example 1: 4,4,4-trifluoro-1-[3-(2,2,2-trifluoroethoxy)-1-oxa-2,8-diazaspiro[4.5]decane- 2-en-8-yl]butan-1-one
[0237]
[0238] Intermediate 2 (200 mg, 0.59 mmol) was dissolved in 2 mL DCM, and TFA (2 mL) was added dropwise at 0 °C. The mixture was stirred at 0°C for 10 minutes. A saturated solution of sodium bicarbonate was added to the reaction mixture, and the product was extracted with EtOAc. Anhydrous MgSO for organic layer 4 Dry, filter, and concentrate to dryness to give a yellow residue.
[0239] To a solution of DIPEA (ALFA-AESAR, 0.3 ml, 1.76 mmol) in DMF (5 mL) was added HBTU (SIGMA-ALDRICH, 669 mg, 1.76 mmol) and 4,4,4-trifluorobutyric acid at 0 °C under argon (ALFA-AESAR, 125 mg, 0.88 mmol). The above residue was then added and the mixture was stirred overnight at room temperature under argon. DMF was then removed under vacuum. by HPLC (constant gradient, CAN / H 2 (Ammonium formate pH 3.8: 36 / 64) purified the residue to give the title compound (1...
Embodiment 5
[0247] Example 5 and Intermediate 19: 1-(3-Bromo-1-oxa-2,8-diazaspiro[4.5]dec-2-en-8-yl)-4,4,4-trifluoro Butan-1-one
[0248]
[0249] To a suspension of Intermediate 7 (9.3g, 42mmol) and sodium bicarbonate (ALFA-AESAR, 35.3g, 420mmol) in EtOAc (300ml) was added dibromoformaldoxime (COMBI-BLOCKS, 10.2g, 50mmol), and The reaction mixture was stirred at room temperature for 2 days. Celite was then added, the resulting slurry was filtered under vacuum and washed with EtOAc, the solvent was evaporated and the residue (about 20 g) was purified by flash chromatography (Si SNAP 340, CyHex / EtOAc from 8 / 2 to 1 / 1) The title compound (12.23 g, 85%) was obtained as a colorless oil. 1 H NMR (400MHz, CDCl 3 )δppm:4.32-4.20(m,1H),3.69-3.60(m,1H),3.59-3.47(m,1H),3.31-3.20(m,1H),3.00(s,2H),2.67-2.43( m,4H),2.07-1.96(m,2H),1.78-1.68(m,2H).[ES+MS]m / z 343,345(MH + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com