A kind of compound, covalent organic framework material and its preparation method and application
A covalent organic framework and compound technology, which is applied in the preparation of carbon-based compounds, organic compounds, alkali metal compounds, etc., can solve the problems of cumbersome preparation methods, poor chemical stability and thermal stability of materials, and achieve simple preparation methods, Effect of high chemical stability and thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Synthesis of compound 5-(2-propynyloxy)isophthalaldehyde:
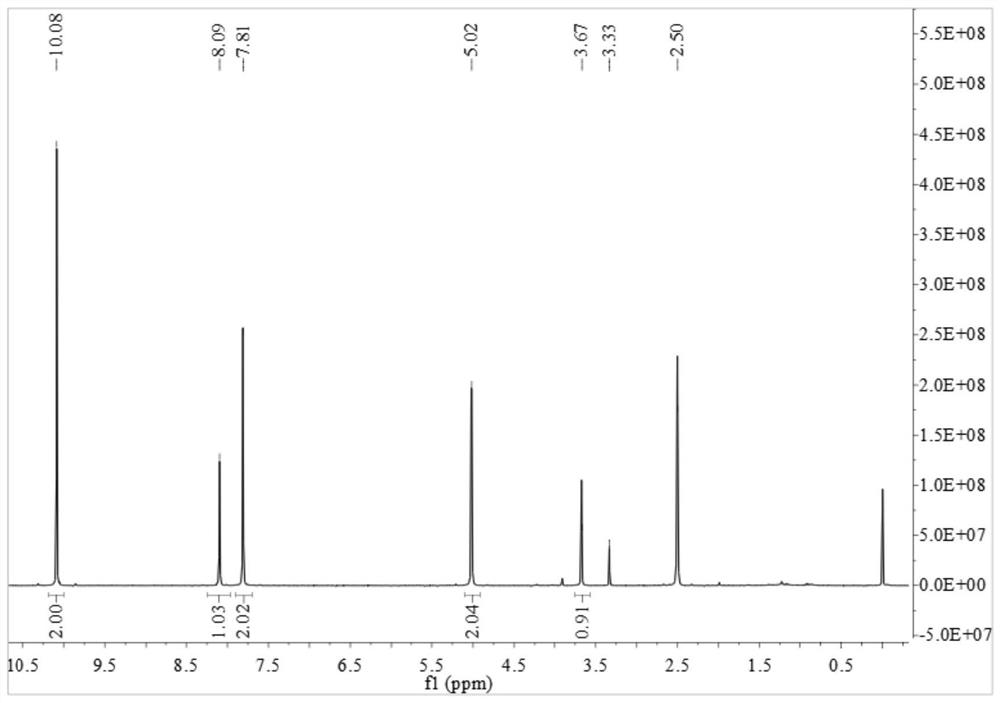
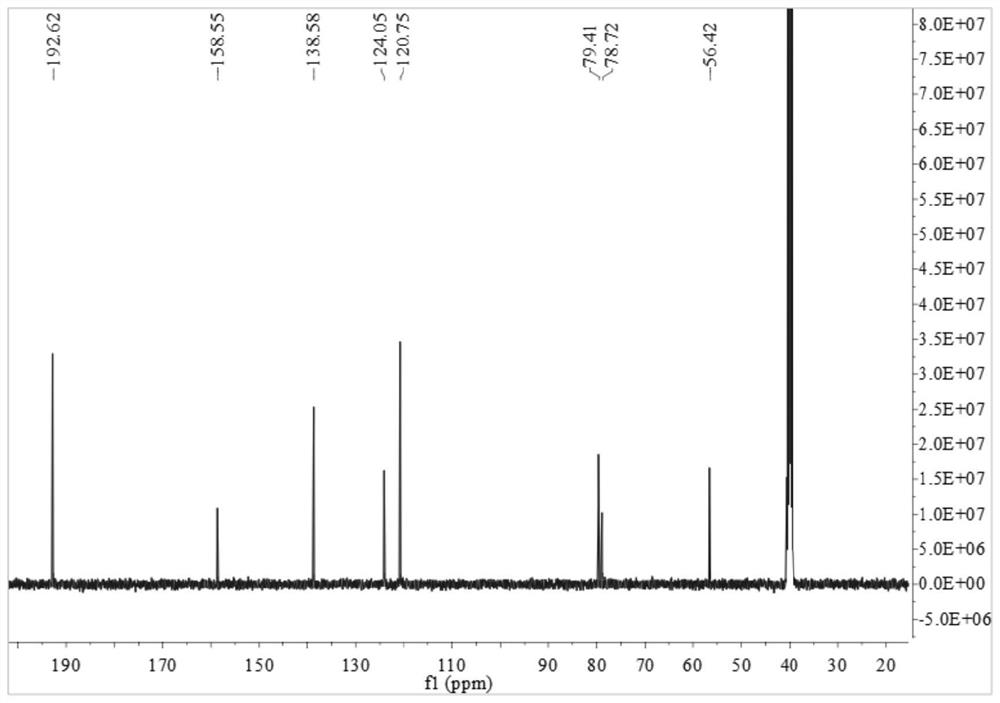
[0049] (1) 5-hydroxyisophthalaldehyde (0.81g, 5.4mmol), potassium carbonate (0.75g, 5.4mmol) and anhydrous tetrahydrofuran (120ml) were added, heated to reflux for 30 minutes and then added 3-bromopropyne (2.5 ml), the reaction was refluxed for 3 days. Cool to room temperature and filter. After distillation under reduced pressure, it was purified by column chromatography (petroleum ether / ethyl acetate=4 / 1, volume ratio) to obtain 0.82 g of white flocculent 5-(2-propynyloxy)isophthalaldehyde aniline, which produced Rate: 81%. 1 H NMR (d 6 -DMSO,400MHz):δ=3.67(t,1H),5.02(d,2H),7.81(d,2H),8.09(s,1H),10.08(s,2H)ppm. 13 C-NMR (d 6 -DMSO, 400MHz): 56.42, 78.72, 79.41, 120.75, 124.05, 138.58, 158.55, 192.62. Its H NMR and C NMR spectra are as follows figure 1 and figure 2 shown.
[0050] The reaction is as follows:
[0051]
Embodiment 2
[0053] To a 10 ml tube was added 5-(2-propynyloxy)isophthalaldehyde (11.3 mg, 0.06 mol) and 1,3,5-tris-(4-aminophenyl)benzene (14.1 mg, 0.04 mmol) Ethanol (1 ml) was added to obtain a pale yellow precipitate, 0.2 ml of acetic acid solution was added to the above mixed solution, and the tube was sealed. Three freeze-thaw cycles were performed and heated in a 120°C oil bath for 3 days. The reaction was completed, centrifuged to obtain a light yellow precipitate, the precipitate was washed three times with tetrahydrofuran, and then fully washed with a Soxhlet extractor (acetone, tetrahydrofuran), and vacuum-dried at 120 degrees Celsius for 12 hours to obtain 11 mg based on 5-(2-propynyloxy) Covalent organic framework materials of isophthalaldehyde building blocks (denoted as BPPA-COF, where Represents repeating units), and the infrared spectrum of covalent organic framework material BPPA-COF is shown in Figure 4 As described, the SEM image is as Figure 5 As shown, the TEM i...
Embodiment 3
[0056] Example 3 Stability Experiment
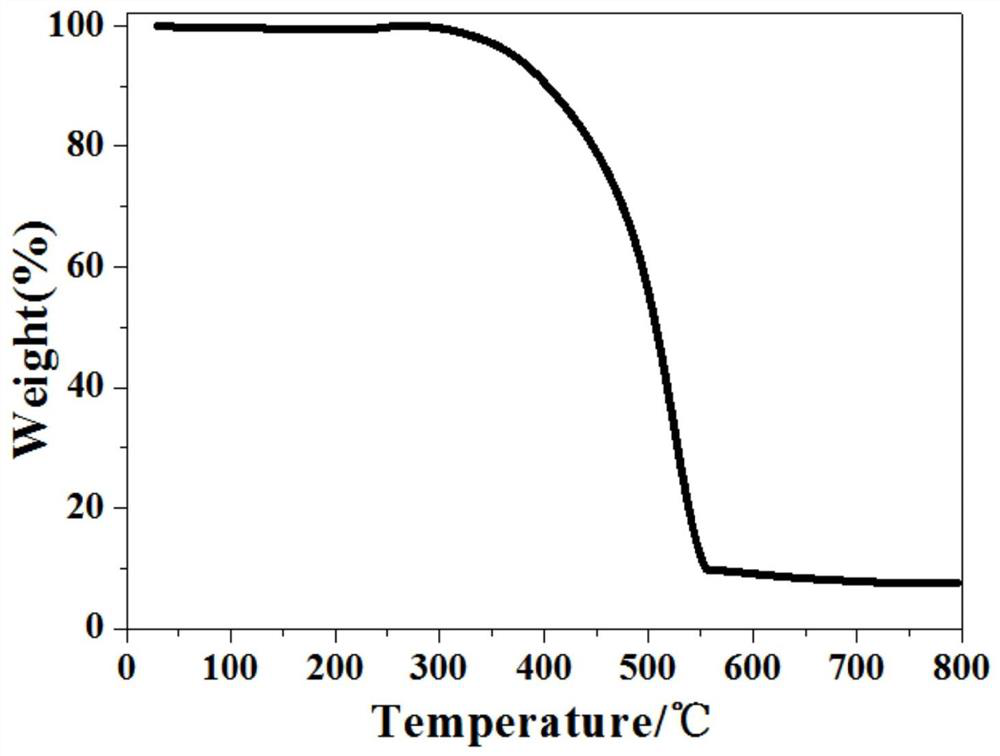
[0057] The BPPA-COF in Example 2 was soaked in an organic solution of N,N-dimethylformamide, methanol, ethanol, acetone, and tetrahydrofuran for three days, and then dried by centrifugation, and then the infrared absorption spectrum was measured, as shown in the figure. The position of each functional group peak has not changed, which is consistent with the initial one. From the thermogravimetric analysis curve in the figure, it can be seen that the BPPA-COF will have significant weight loss at 300 °C, indicating that the thermal stability is good.
[0058] The covalent organic framework material prepared in Example 2 is used for modification, and the thiophenol group containing azide is connected through the click reaction (as shown in the figure below). The experiment shows that it can adsorb mercury ions in water.
[0059]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com