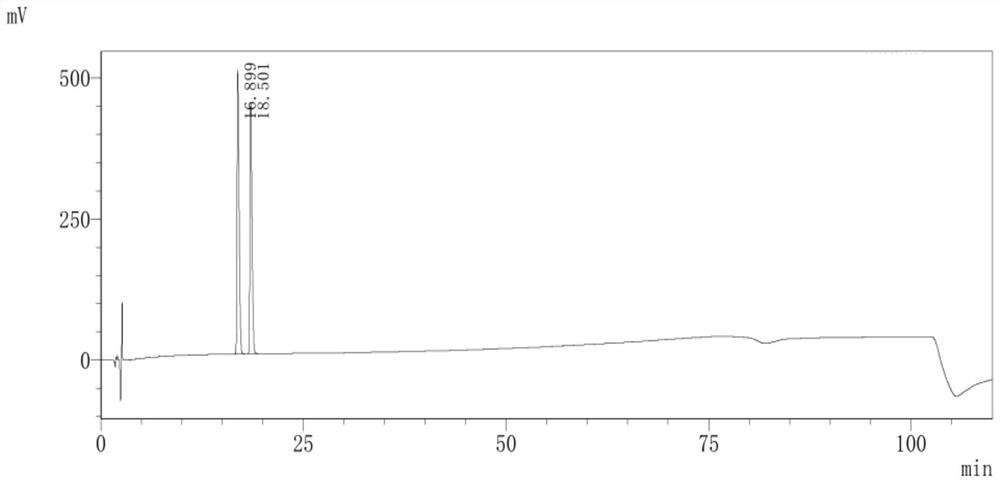
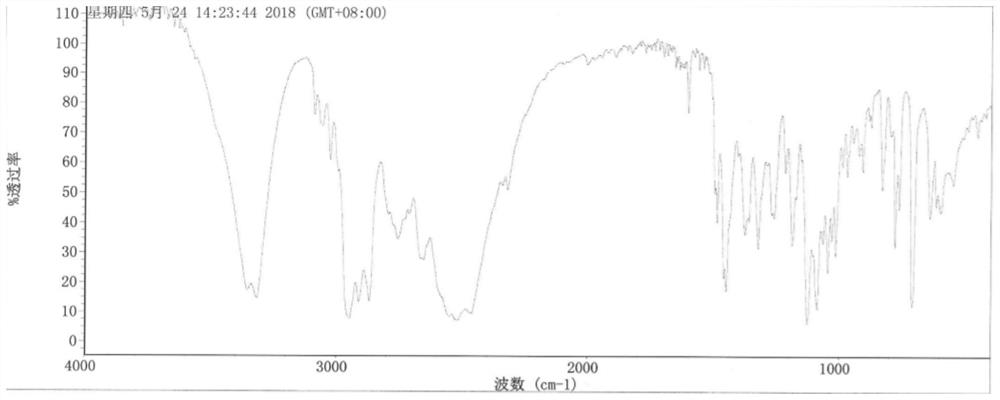
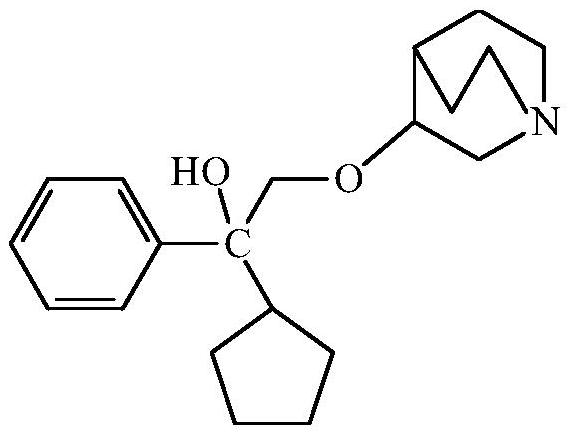
Purification of 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy)quinuclidine and preparation method of penehyclidine hydrochloride
A technology of phenylethoxy and purification method, which is applied in the field of purification of 3-quinuclidine and penehyclidine hydrochloride preparation, can solve the problem of lack of target product impurity removal and purification process, low purity and yield, and poor product quality. Complex operation and other problems, to achieve the effect of low cost, simple purification method and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1-a kind of purification method of 3-(2-cyclopentyl-2-hydroxyl-2-phenylethoxy)quinuclidine, comprises the steps:
[0037] (1) Mix 3-quinuclidinol with dimethyl sulfoxide solution, and add sodium hydride in turn to stir the reaction, add dropwise 1,1-phenylpentyl oxirane solution to react and add dropwise saturated sodium chloride The solution terminates the reaction to obtain a reaction solution; wherein, the mol ratio of 1,1-phenylpentyl oxirane solution, 3-quinuclidinol and sodium hydride is 1:1:1;
[0038] (2) Add purified water to the reaction solution and stir, add ethyl acetate for extraction, separate the ethyl acetate layer, and collect the organic phase; after the organic phase is washed with pure water and saturated sodium chloride solution successively, back-extract with hydrochloric acid solution, collect the water layer;
[0039] (3) Adjust the pH value of the aqueous layer to 5-7 with an alkaline solution, wash with ethyl acetate, adjust the pH...
Embodiment 2
[0041] Embodiment 2-a kind of purification method of 3-(2-cyclopentyl-2-hydroxyl-2-phenylethoxy)quinuclidine, comprises the steps:
[0042] (1) Stir and mix 3-quinuclidinol and dimethyl sulfoxide solution at room temperature at 145-155 rpm for 5-10 minutes, add sodium hydride at room temperature and stir for 25-35 minutes, then heat up Continue constant temperature stirring at 60°C for 1~1.5h; and control the temperature below 20°C, add 1,1-phenylpentyl oxirane solution dropwise, after the dropwise addition is completed within 25~35min, raise the temperature to 70°C for reaction 3.5 to 4.5 hours; lower the temperature to below 20°C, add a saturated sodium chloride solution dropwise to terminate the reaction, and obtain a reaction liquid; among them, 1,1-phenylpentyl oxirane solution, 3-quinuclidinol and sodium hydride The molar ratio is 1:2.5:1.5;
[0043] (2) Add purified water to the reaction solution and stir, add ethyl acetate for extraction, separate the ethyl acetate la...
Embodiment 3
[0046] Embodiment 3-a kind of penhyclidine hydrochloride preparation method comprises the steps:
[0047] (1) Add tert-butyl methyl ether to the oil of 3-(2-cyclopentyl-2-hydroxy-2-phenylethoxy)quinuclidane prepared in Example 2, and heat to 55-65°C After dissolving, cool down to -5~0°C, add hydrogen chloride ethyl acetate solution dropwise under stirring condition until the pH value of the solution is 2~3, slowly add tert-butyl methyl ether, and stir at room temperature to precipitate a solid;
[0048] (2) Filter the precipitated solid, wash it with tert-butyl methyl ether, and dry it under vacuum at 65-75° C. for 2-3 hours to obtain penhyclidine hydrochloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com