Anti-L1-CAM antibodies and uses thereof
An L1-CAM, antibody technology, applied in the direction of antibodies, antibody medical components, antibody mimics/stents, etc., can solve problems such as bystander toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
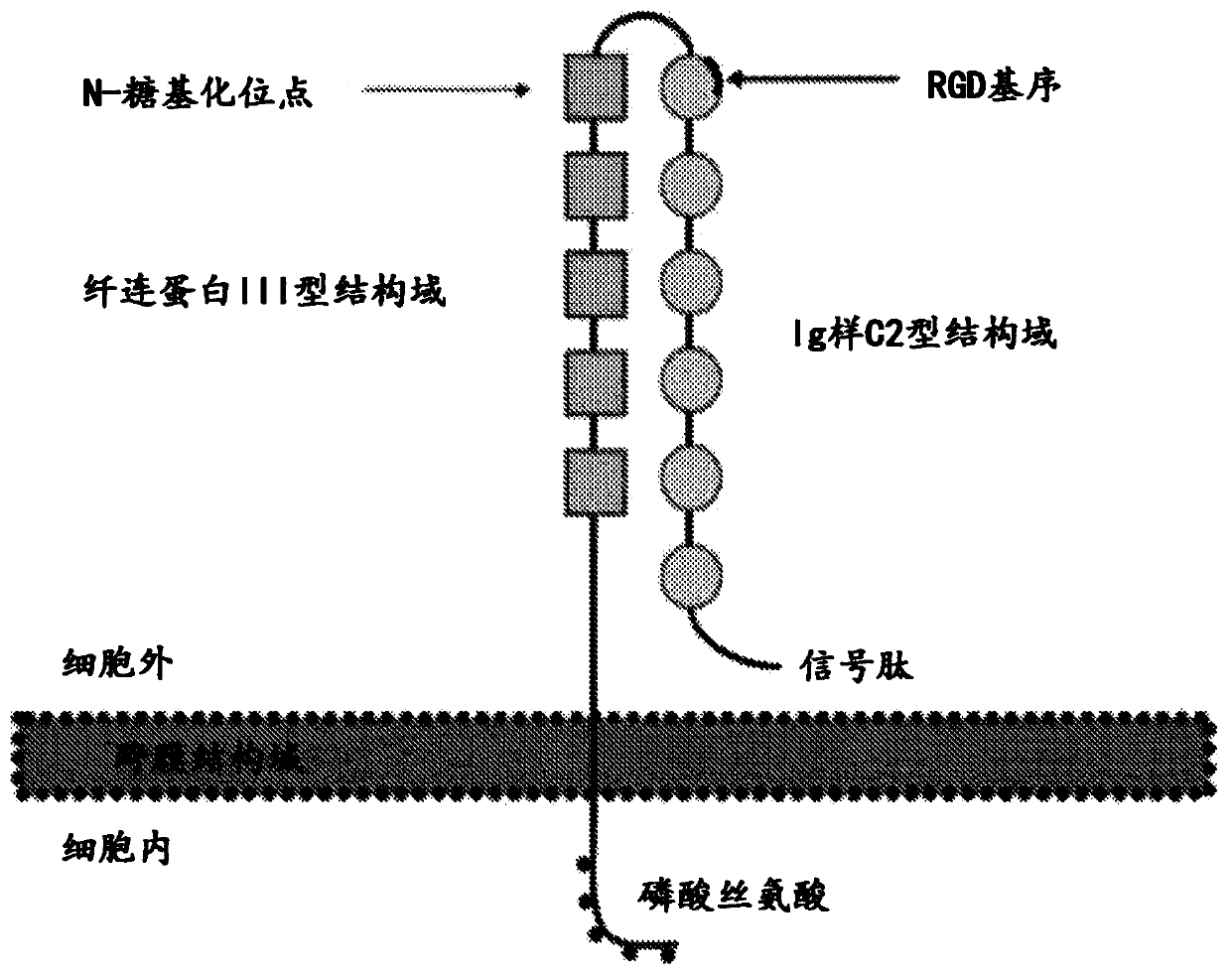
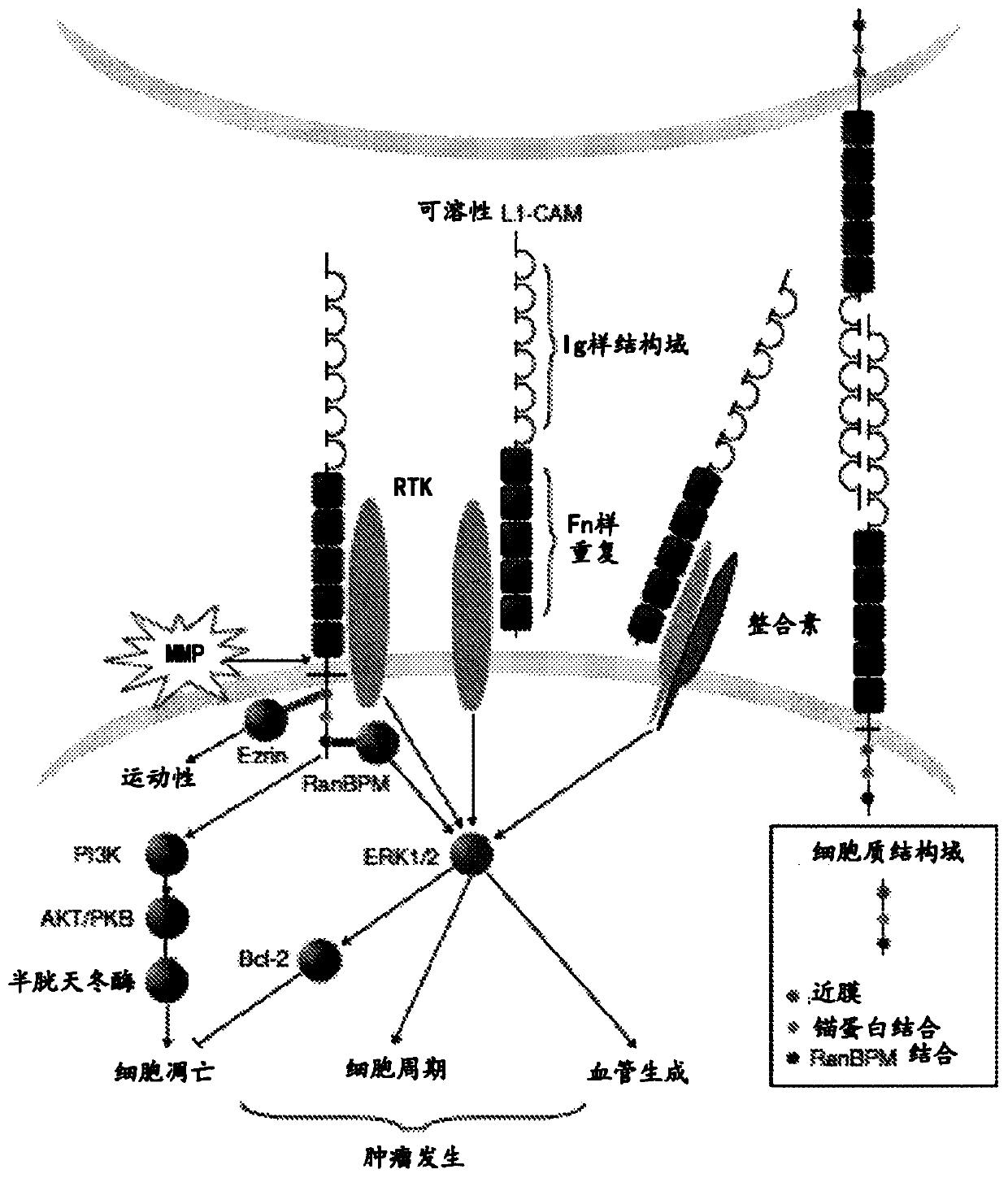
[0222] Preparation of polyclonal antisera and immunogens. Methods for producing antibodies or antibody fragments of the present technology generally include treating a subject (usually a non-human subject, such as a mouse or rabbits) for immunization. Suitable immunogenic preparations may contain, for example, recombinantly expressed L1-CAM protein or chemically synthesized L1-CAM peptide. Using standard techniques for polyclonal and monoclonal antibody preparation, the ECM of the L1-CAM protein or a portion or fragment thereof can be used as an immunogen to generate anti-L1-CAM antibodies that bind to the L1-CAM protein or a portion or fragment thereof.
[0223] The full length L1-CAM protein or fragments thereof can be used as fragments, as immunogens. 在一些实施方案中,L1-CAM片段包含氨基酸序列PKETVKPVEVEEGESVVLPCNPPPSAEPLRIYWMNSKILHIKQDERVTMGQNGNLYFANVLTSDNHSDYICHAHFPGTRTIIQKEPID(SEQ ID NO:74)(L1-CAM的Ig样C2型第2结构域)或TQITQGPRSTIEKKGSRVTFTCQASFDPSLQPSITWRGDGRDLQELGDSDKYFIEDGRLVIHSLDYSDQGNYSCVAST...
Embodiment 1
[0355] Example 1: Materials and Methods for Producing and Characterizing Anti-L1-CAM Antibodies of the Present Technology
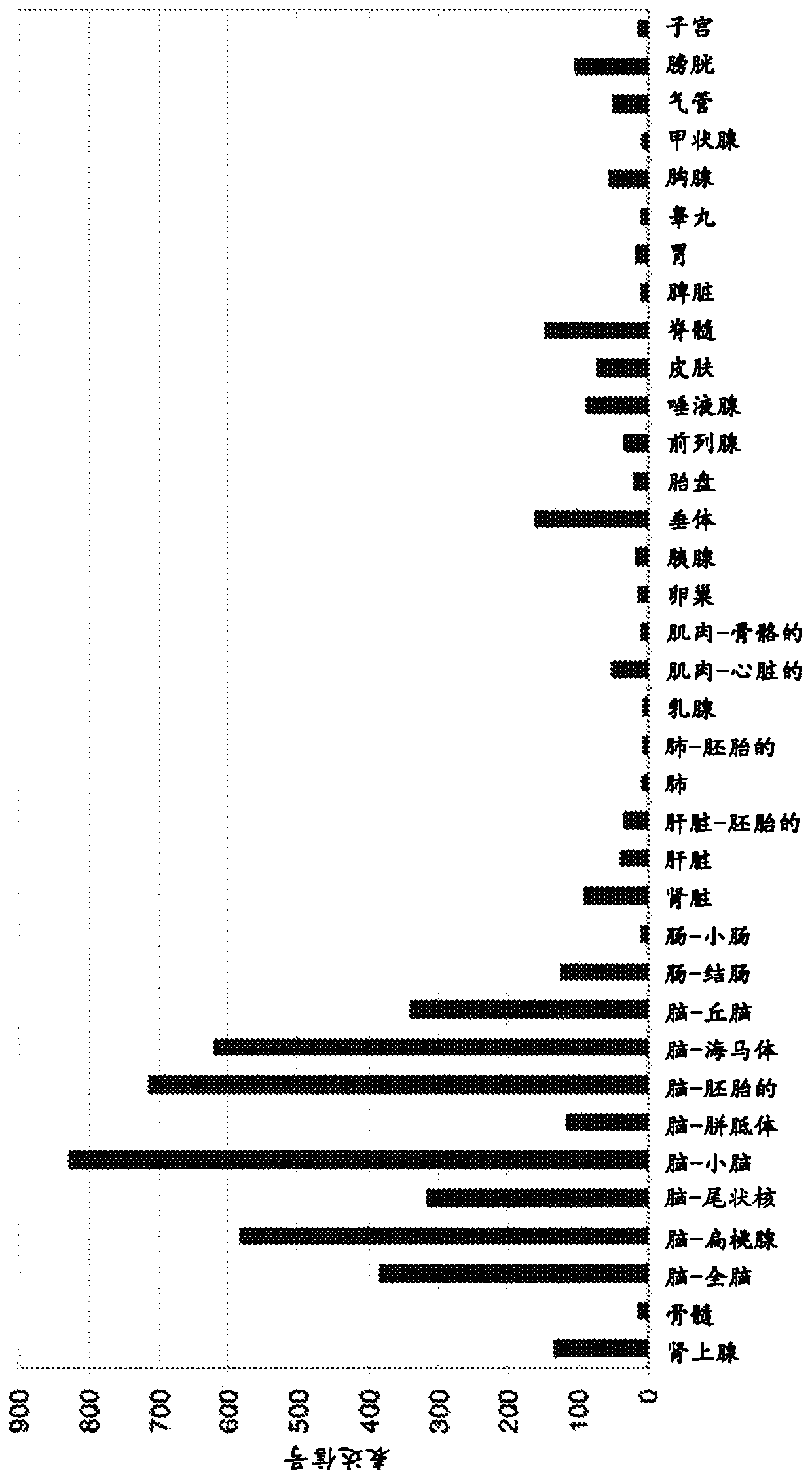
[0356] Immunohistochemistry (IHC). The use of human tumor and normal tissues for IHC was approved by the Institutional Review Board of Memorial Sloan-Kettering Cancer Center. Five to seven micron sections of snap-frozen tissue were fixed in acetone at -20°C for 30 minutes. Endogenous biotin-binding activity was blocked by sequential treatment with avidin and biotin (Carrier Avidin-Biotin Blocking Kit; Invitrogen, Carlsbad, CA) for 20 min, followed by Each was blocked with 10% horse serum for 1 hour at room temperature. Sections were then stained sequentially with primary antibody, biotinylated horse anti-mouse IgG (H+L) (Vector Laboratories, Inc., Burlingame, CA) and avidin-biotin complex (Vectastain ABC kit, VectorVector Laboratories, Inc., Burlingame, CA) for 60 min at room temperature with washes between each reaction. Subsequently, sections were...
Embodiment 2
[0375] Example 2: Structure of the humanized anti-L1-CAM antibody of the present technology
[0376] Humanized E71 and E72 anti-L1-CAM antibodies were generated. Sequence design is based on human IgG homology calculations while retaining key mouse amino acid residues. Image 6 with Figure 7 The V of murine E71 is shown separately H and V L (represented as SEQ ID NO: 1 and 3) and the V of mouse E72 H and V L (Denoted as SEQ ID NO: 5 and 7). Mouse E71 (see Image 6 ) and E72 (see Figure 7 ) heavy and light chain CDRs were grafted onto the human IgG1 framework based on their homology to the human framework. For E71, the homologous human sequence used for the heavy chain was IGHV7-4-1 (SEQ ID NO:2), and the homologous human sequence used for the light chain was IGKV-58 (SEQ ID NO:4) ( Image 6 ). For E72, the homologous human sequences used for the heavy and light chains were IGHV1-2*02|66.3|IGHJ4*01|85.7 (SEQ ID NO: 6) and IGKV1-NL1*01|73.7|IGKJ2*02| 81.8 (SEQ ID N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com