Metal organic frameworks appended with cyclic diamines for carbon dioxide capture
An organic framework and organic technology, applied in the direction of magnesium organic compounds, other chemical processes, chemical instruments and methods, etc., can solve problems such as interference adsorption/desorption kinetics adsorption mechanism, diamine loss, degradation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] I. Introduction
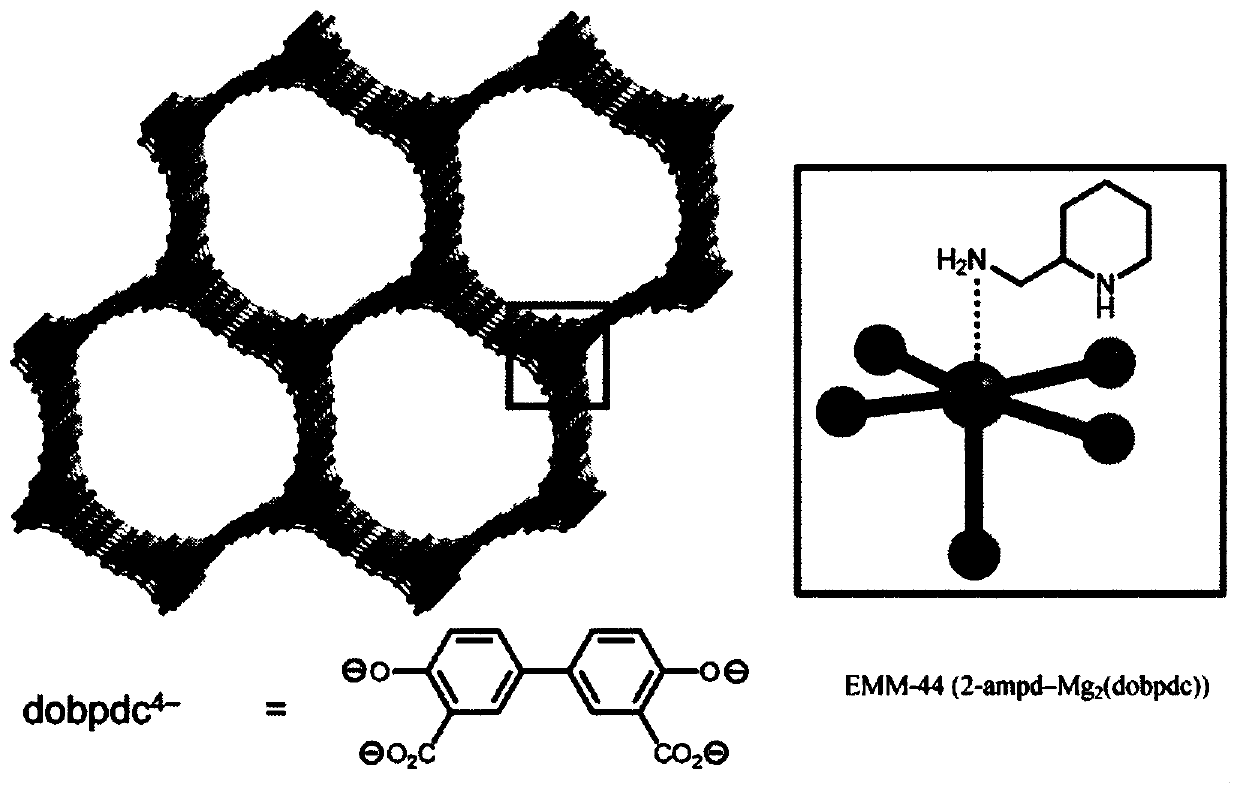
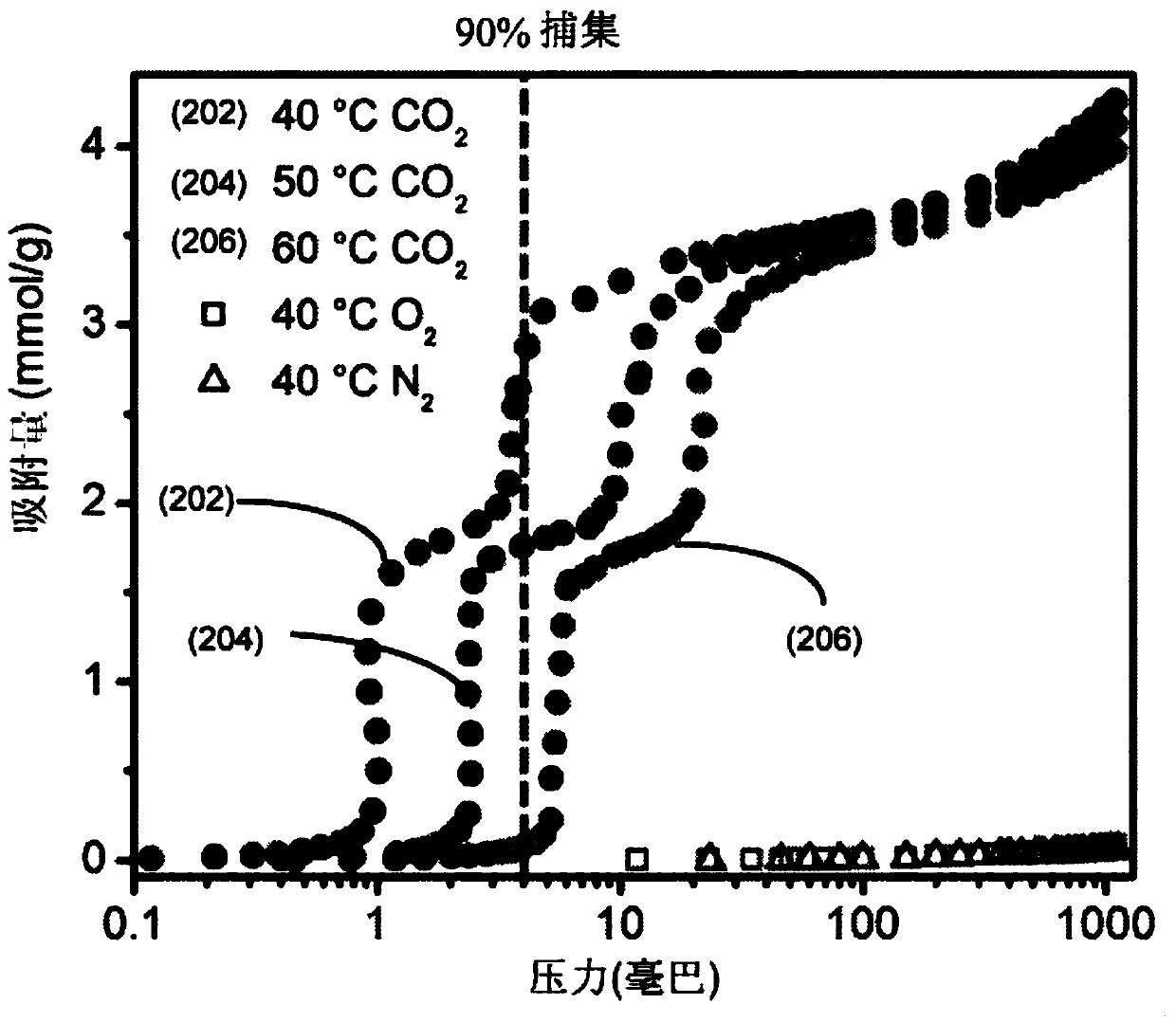
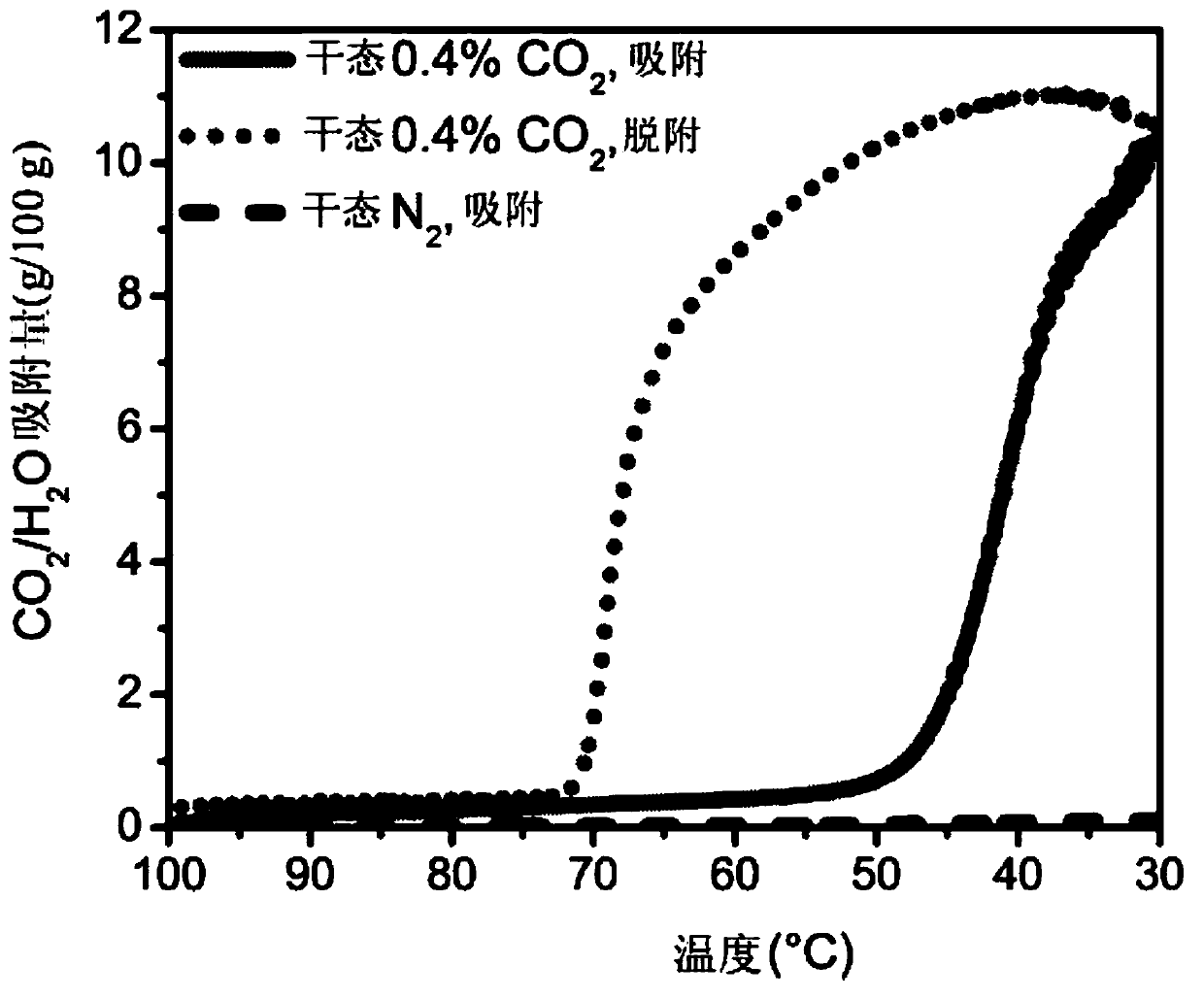
[0031] Attachment of the diamine 2-(aminomethyl)piperidine (2-ampd) to metal-organic frameworks Mg 2 (dobpdc)(dobpdc 4- =4,4'-biphenyldioxide-3,3'-diformate), Mg 2 (dotpdc)(dotpdc 4- = 4,4"-dioxide-[1,1':4',1"-terphenyl]-3,3"-diformate) or Mg 2 (pc-dobpdc)(pc-dobpdc 4- = open Mg of biphenyl dioxide-4,4'-dicarboxylate) 2+ sites, yielding promising sorbents EMM-44, EMM-45, and EMM-46, respectively, for capturing CO from flue emissions from natural gas-fired power plants 2 ( figure 1 ). The sorbent has a number of properties that make it promising for capturing carbon from natural gas-fired power plants.
[0032] Before the present invention is described in greater detail, it is to be understood that this invention is not limited to particular embodiments described herein, as such embodiments may vary. It is also to be understood that terminology used herein is for the purpose of describing particular embodiments only and that terminology is not...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com