Process to prepare cyclic oligomer and cyclic oligomer obtainable thereby and process to polymerize SAME
A technology of oligomers and cyclic polyesters, which is applied in the preparation of carboxylates by oligomerization, climate sustainability, sustainable manufacturing/processing, etc., and can solve problems such as degradation and discoloration of aliphatic oligomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
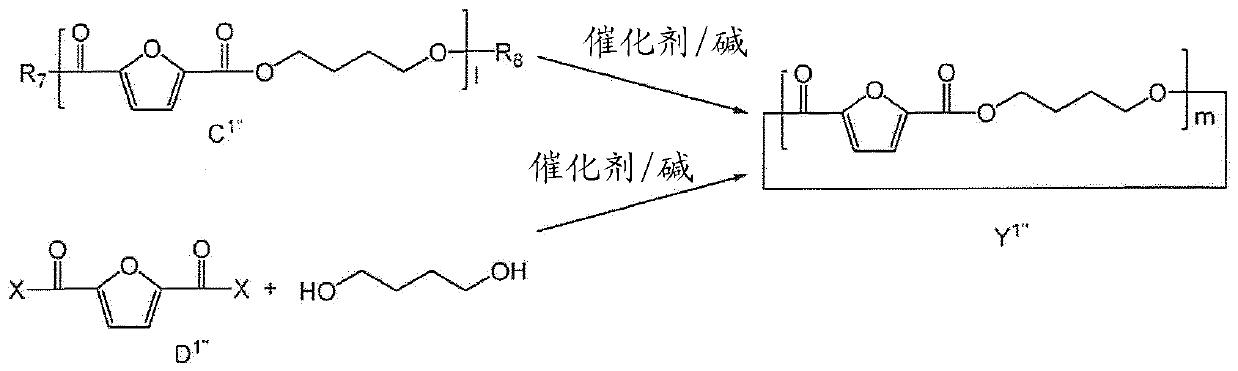
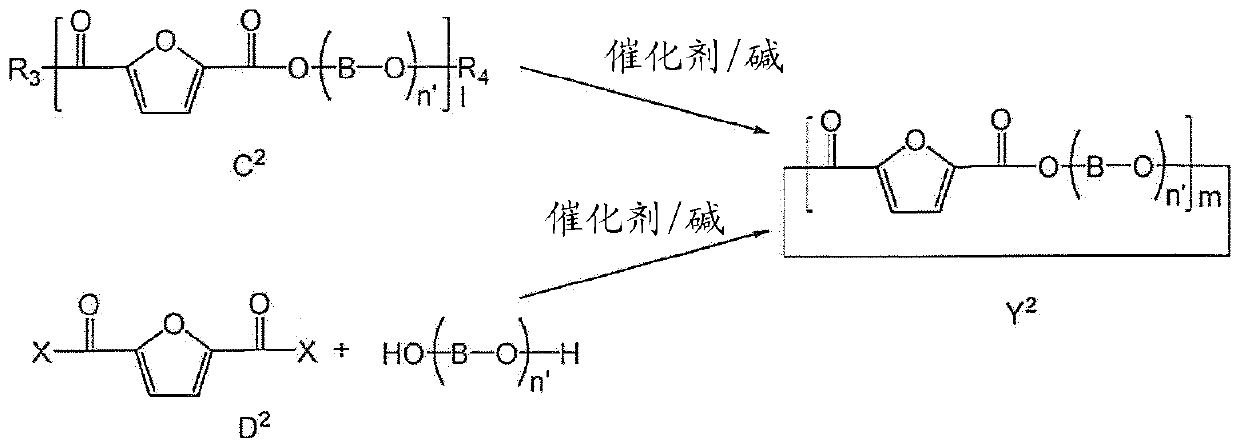
[0184] Embodiment 1: be used to produce the cyclic polyester oligomer composition of PEF (Y 1' implementation plan)
[0185] In this embodiment, it is described image 3 Preparation of cyclic polyester oligomers shown in , which can then be subsequently used to prepare PEF, poly(ethylene 2,5-furandicarboxylate). 40 g of me-FDCA was loaded with 20 ml of EG into a glass reactor equipped with a stirrer. The reaction was carried out under an inert atmosphere at an initial temperature of 140°C in the presence of 0.50 g of catalyst (Bu2SnO) and gradually heated to a final temperature of 180°C. After 1 hour of reaction, the pressure was reduced to 700 mbar; after 40 minutes the pressure was reduced again to 400 mbar and after 30 minutes to 200 mbar. Finally the pressure is gradually reduced up to 10 mbar. The temperature was raised to 200°C and the system was kept under these conditions for 2 hours. The system was allowed to cool to room temperature and the solid product was r...
Embodiment 2
[0190] Embodiment 2: by cyclic polyester oligomer composition (Y 1' ) to produce PEF: in the presence of a small amount of plasticizer and catalyst Down
[0191] In this example, the cyclic oligomer Y of Example 1 was 1' (m=2) Reacted as in Comparative Example 1, but with the presence of cyclic stannoxane as catalyst at a concentration of 0.1 mol % per mol of cyclic oligomer repeating units. In this case, conversions greater than 95% were achieved within 20 minutes.
[0192] Figure 5 Comparative data showing the conversion of cyclic PEF dimers using lower (1 / 3 v / m) and higher (2 / 3 v / m) concentrations of tetraethylene glycol dimethyl ether plasticizer .
[0193] In other polymerization experiments, other metal oxide catalysts such as Sb 2 O 3 or Bi 2 O 3 Compare with tin-based catalysts. observed use of Sb 2 O 3 or Bi 2 O 3 The polymer produced was more water-white in appearance than the slightly yellowish-brown color obtained with the tin-based catalyst.
Embodiment 3
[0194] Embodiment 3: by cyclic polyester oligomer composition (Y 1' ) to produce PEF: in the presence of a higher amount of plasticizer No catalyst
[0195] In this example, the cyclic oligomer Y of Example 1 1'(m=2) React as in Comparative Example 1, with 240 uL tetraethylene glycol dimethyl ether per 180mg cyclic oligomer Y 1' Higher concentrations use tetraethylene glycol dimethyl ether as a plasticizer. In this case, greater than 95% conversion was achieved within 60 minutes at all temperatures.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com