Crystal form of alkynylpyridine prolyl hydroxylase inhibitor and preparation method thereof
A crystal form and compound technology, which is applied to the crystal form of alkynylpyridine prolyl hydroxylase inhibitors and the field of preparation thereof, can solve the problems of poor fluidity, poor product stability, difficulty in production amplification and the like, and achieve HPLC purity changes. Small size, good crystal stability, and stable production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
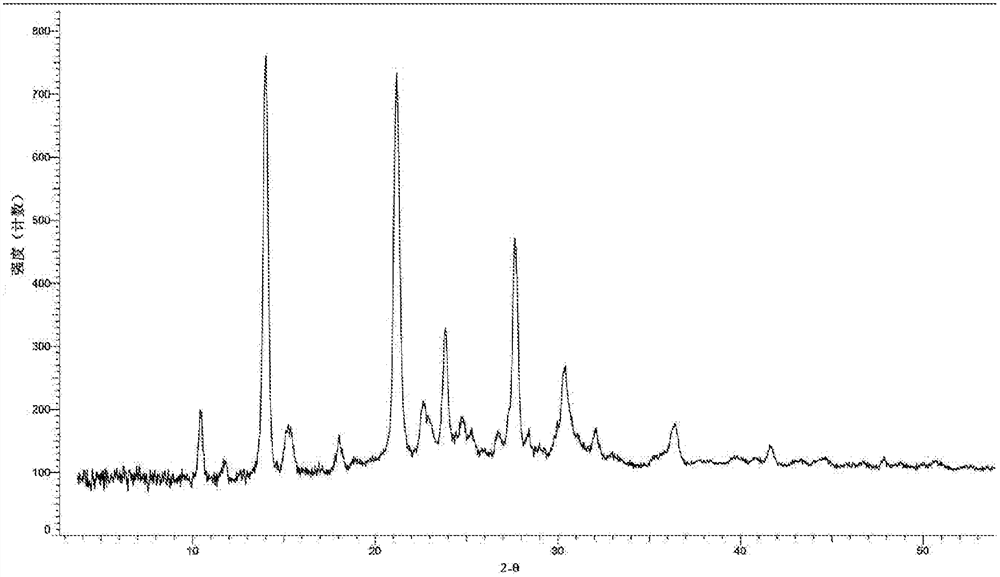
[0106] Weigh 10 mg of the compound represented by formula (I) (prepared according to the method disclosed in WO2017059623) into a reaction flask, add 5 ml of dimethyl sulfoxide to dissolve, leave it at room temperature to evaporate to dryness, and obtain about 9 mg of a light yellow solid. The X-ray diffraction pattern of this crystalline sample is shown in figure 1 , whose DSC spectrum is shown in Figure 7 , define this crystal form as A crystal form, and its characteristic peak positions are shown in the table below:
[0107] Table 1. Characteristic peaks of crystal form A
[0108]
[0109]
Embodiment 2
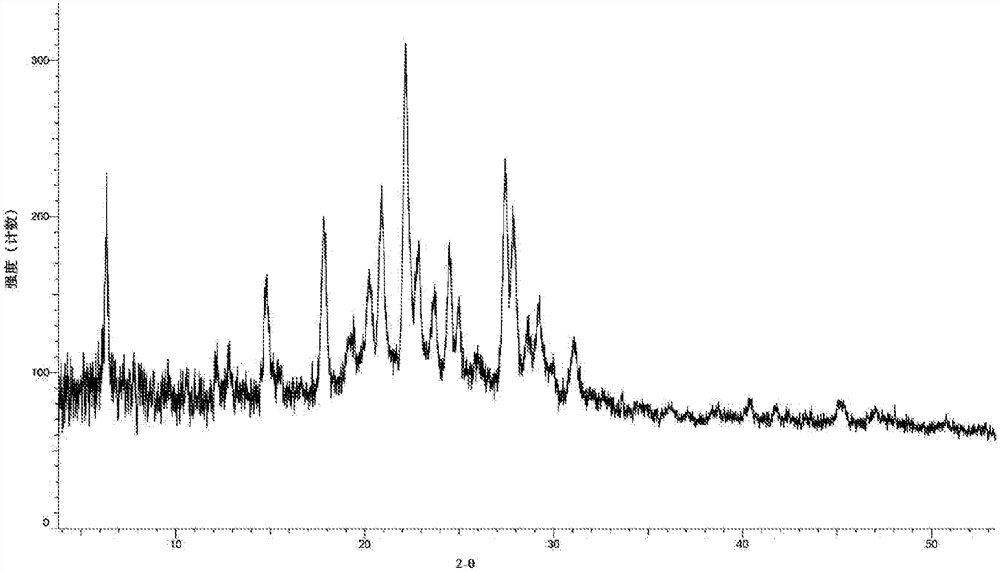
[0111] Weigh 10 mg of the compound represented by formula (I) into a reaction flask, add 5 ml of dichloromethane, place at room temperature and 50° C. for beating for 3 days, filter, and vacuum dry at 40° C. for 2 hours. Both obtained about 8 mg of a light yellow solid, and the X-ray diffraction patterns of the two were consistent. The X-ray diffraction pattern of this crystalline sample is shown in figure 2 , whose DSC spectrum is shown in Figure 8 , define this crystal form as B crystal form, and its characteristic peak positions are shown in the table below:
[0112] Table 2. Characteristic peaks of crystal form B
[0113]
[0114]
Embodiment 3
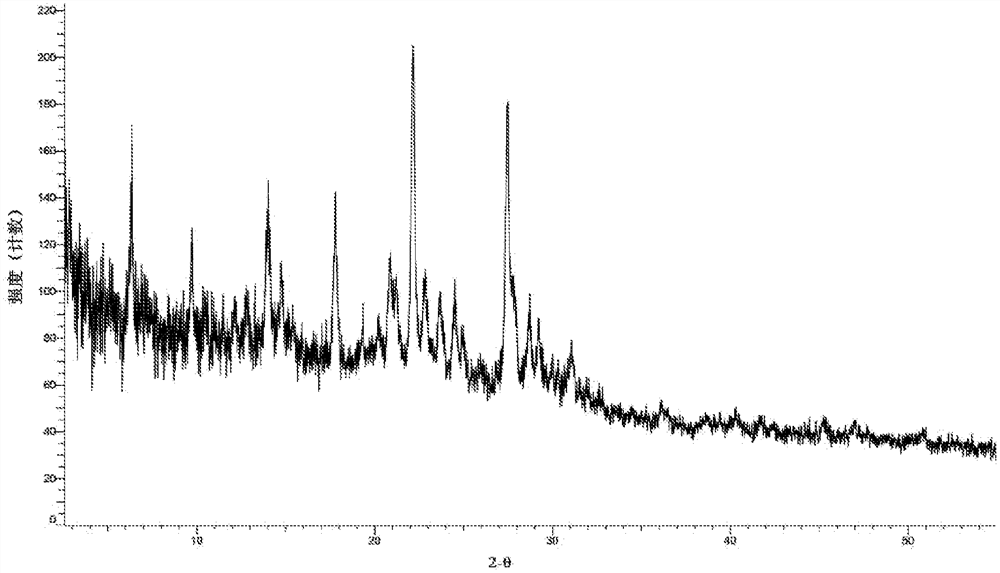
[0116] Take by weighing the compound shown in 80mg formula (I) and join in the reaction bottle, add 40ml methanol / water mixed solvent (V 水 :V 甲醇 = 1:9) and stirred to dissolve, left at room temperature and evaporated to dryness. About 75 mg of light yellow solid was obtained. The X-ray diffraction pattern of this crystalline sample is shown in image 3 , whose DSC spectrum is shown in Figure 9 , define this crystal form as C crystal form, and its characteristic peak positions are shown in the table below:
[0117] Table 3. Characteristic peaks of crystal form C
[0118]
[0119]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com