Crystalline form of free base of imidazoisoindole derivatives and preparation method thereof
A technology of crystallization and crystal form, applied in the disease field with pathological characteristics of tryptophan metabolism pathway, can solve the problems of difficult filtration, fine crystallization, and the stability of crystal form needs to be improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
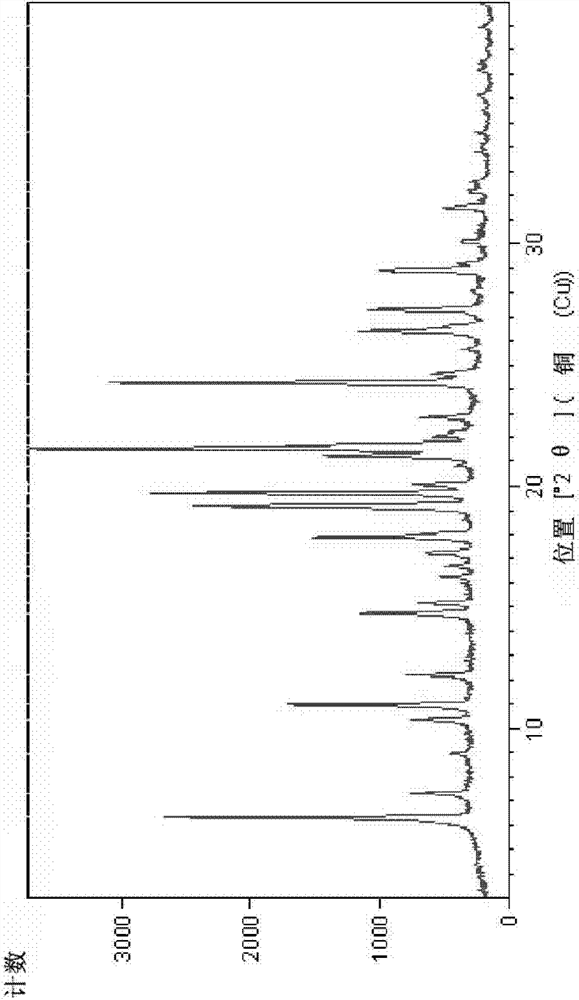
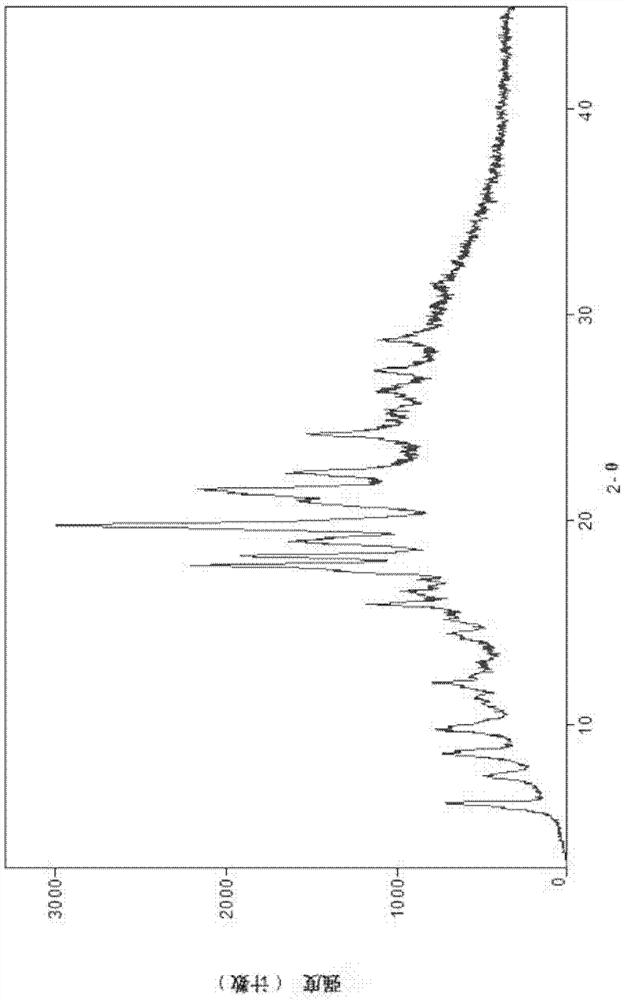
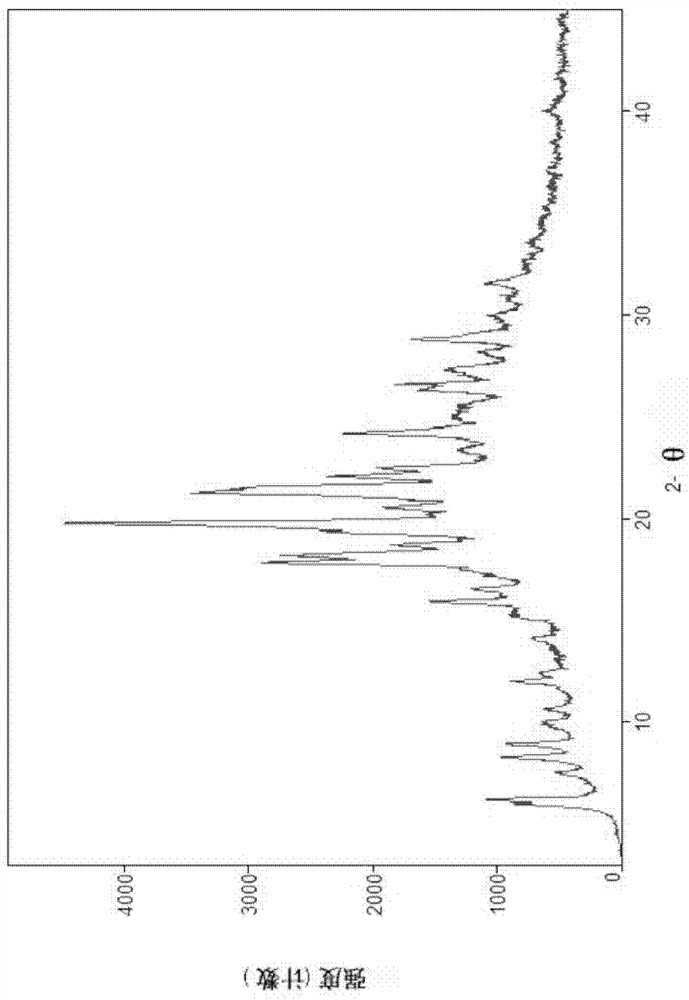
Image
Examples
Embodiment Construction
[0047] The starting material used in the preparation method of the crystal form of the present invention can be any form of the compound represented by formula (I), and the specific form includes but not limited to: amorphous form, any crystalline form, etc.
[0048] In the specification and claims of the present application, unless otherwise specified, the scientific and technical terms used herein have the meanings commonly understood by those skilled in the art. However, for a better understanding of the present invention, definitions and explanations of some related terms are provided below. In addition, when the definitions and explanations of terms provided in this application are inconsistent with the meanings commonly understood by those skilled in the art, the definitions and explanations of terms provided in this application shall prevail.
[0049] The "beating" in the present invention refers to the method of purifying substances with poor solubility in solvents but...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com