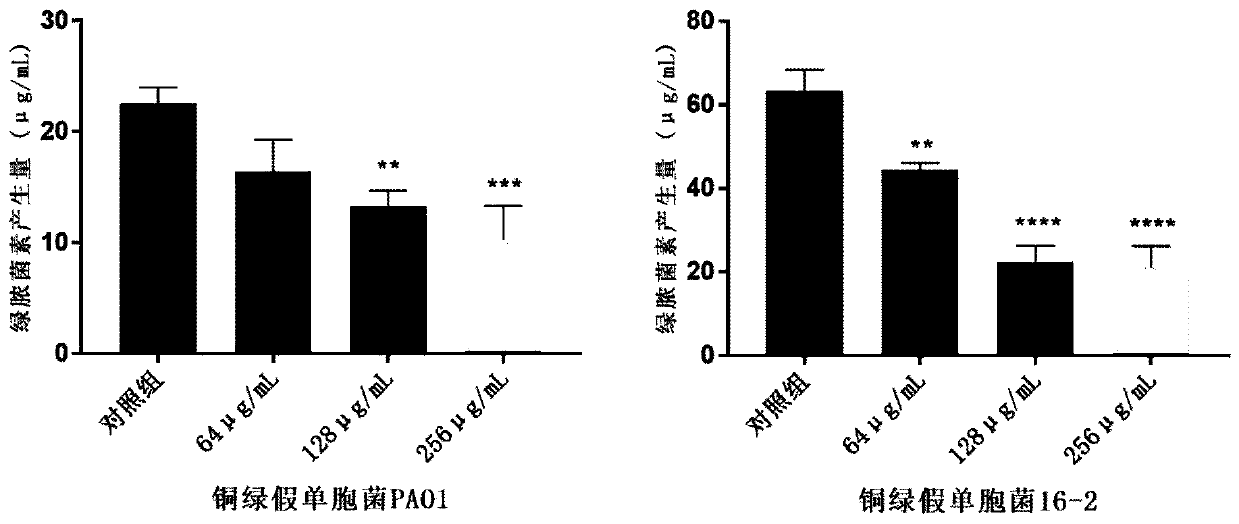
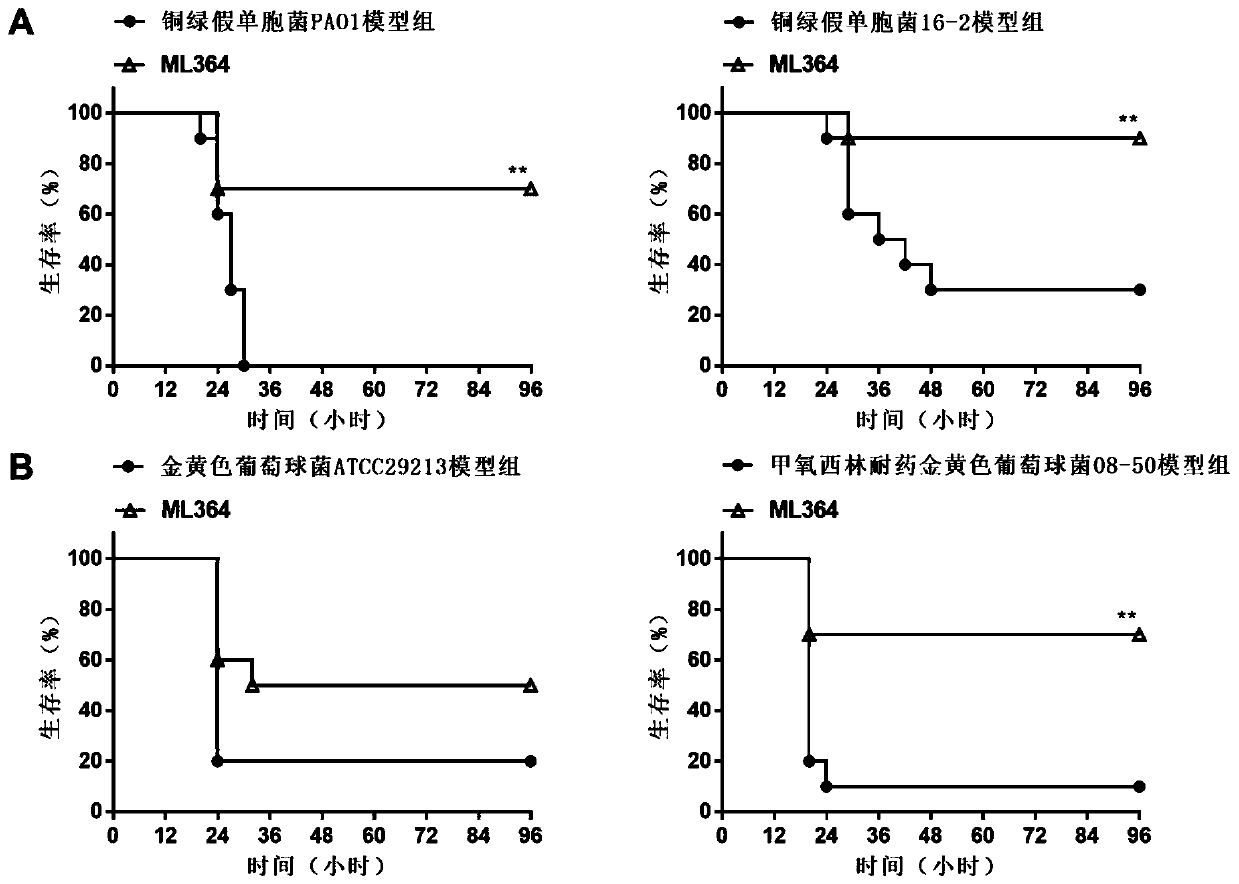
Anti-infection application of thiazole-structure-containing compound
A compound and application technology, which is applied in the field of anti-infection application of compounds containing thiazole structures, can solve problems such as easy drug resistance and high pressure for bacterial survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
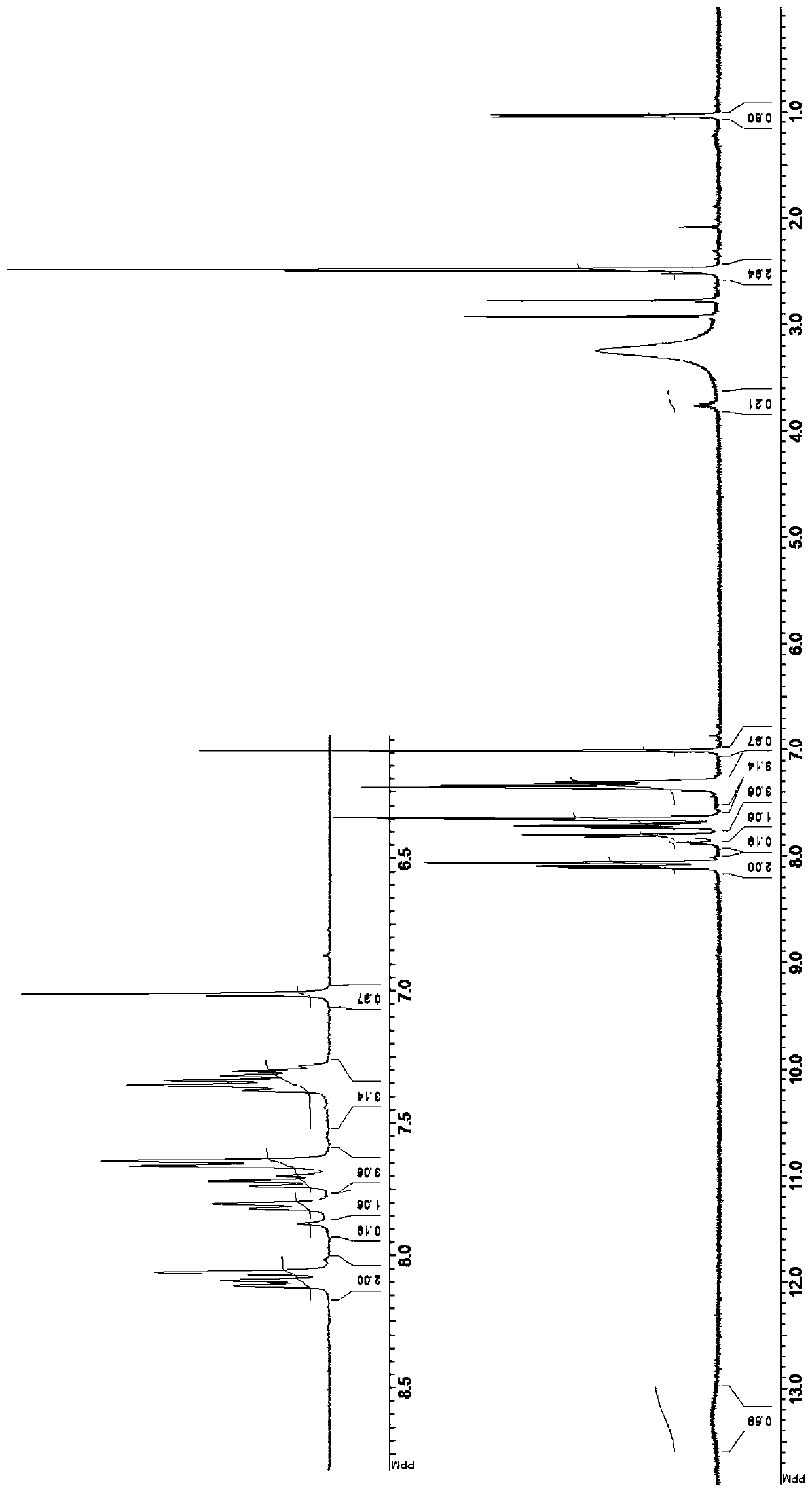
[0176] Synthesis of Example 1.1 Compound ML364 (2-((4-methylphenyl)sulfonylamino)-N-(4-phenylthiazol-2-yl)-4-(trifluoromethyl)benzamide) References: Mindyl I. Davis et al., Small Molecule Inhibition of the Ubiquitin-specific Protease USP2 Accelerates cyclinD1 Degradation and Leads to Cell Cycle Arrest in Colorectal Cancer and MantleCell Lymphoma Models, THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL.291, NO.47, pp. 24628–24640, November 18, 2016. The compounds in Table 1 can be synthesized in a similar manner.
Embodiment 12
[0177] Example 1.2 Synthesis of compound 2-((4-fluorophenyl)sulfonylamino)-N-(4-(4-isopropylphenyl)thiazol-2-yl)benzamide: same as in Example 1.1 method to synthesize the target compound.
[0178]
Embodiment 13
[0179] Synthesis of Example 1.3 Compound 2-((4-fluorophenyl)sulfonylamino)-N-(4-(4-methylphenyl)thiazol-2-yl)benzamide: obtained in Example 1.2 Intermediate 5 and Intermediate 6 were obtained in the same way as the target compound.
[0180]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com