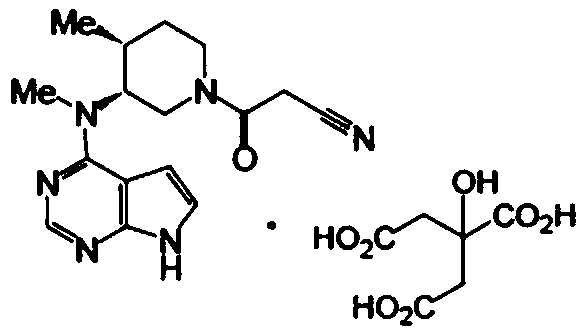
Tofacitinib citrate sustained release tablet and preparation method thereof
A technology of tofacitinib and citric acid is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Exhaust gas and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
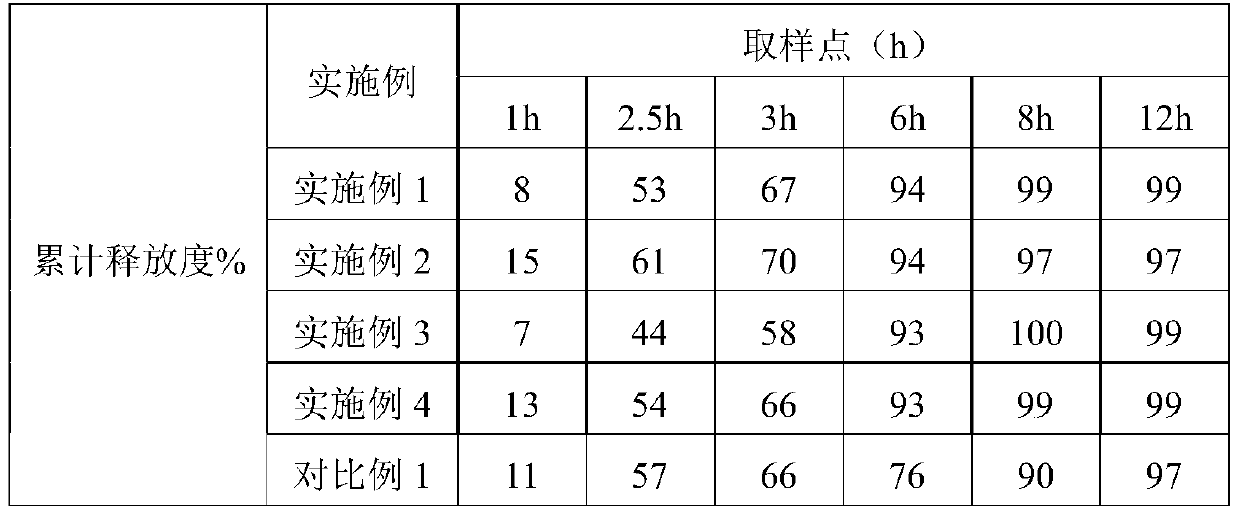
[0019] Embodiment 1: Preparation of Tofacitinib Citrate Sustained Release Tablets:
[0020] Prescription composition of Tofacitinib Citrate Sustained-release Tablets: Specification 11mg (calculated as Tofacitinib)
[0021] Raw materials Dosage g Tofacitinib citrate 8.1 Compritol@888ATO 4.05 HPMC 24.3 MCC / Lactose 63.05 Magnesium stearate 0.5
[0022] Preparation Process:
[0023] (a) Glyceryl behenate is weighed, heated to 65-70°C for melting, and kept warm;
[0024] (b) Continue to keep warm, add tofacitinib citrate at the same time, use an electric stirrer to stir for more than 30 minutes until it melts, and quickly put it into an ice-water mixture to solidify. After it is completely solidified, grind it and pass it through an 80-mesh sieve to obtain the drug-loaded particles;
[0025] (c) Mix the drug-loaded granules with the prescribed amount of HPMC, MCC / lactose (passed through a 100-mesh sieve), and magnesium stearate, and p...
Embodiment 2
[0026] Embodiment 2: Preparation of Tofacitinib Citrate Sustained Release Tablets:
[0027] Prescription composition of Tofacitinib Citrate Sustained-release Tablets: Specification 11mg (calculated as Tofacitinib)
[0028] Raw materials Dosage g Tofacitinib citrate 8.1 Compritol@888ATO 8.1 HPMC 8.1 MCC / Lactose 75.2 Magnesium stearate 0.5
[0029] Preparation process: with embodiment 1.
Embodiment 3
[0030] Embodiment 3: Preparation of Tofacitinib Citrate Sustained Release Tablets:
[0031] Prescription composition of Tofacitinib Citrate Sustained-release Tablets: Specification 11mg (calculated as Tofacitinib)
[0032] Raw materials Dosage g Tofacitinib citrate 8.1 Compritol@888ATO 24.3 HPMC 24.3 MCC / Lactose 42.3 Magnesium stearate 1
[0033] Preparation process: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com