Erythrocyte membrane-encapsulated rapamycin nanoparticle and preparation method and application thereof
A technology of rapamycin and red blood cell membrane, which is applied in the field of rapamycin nanoparticles and its preparation, which can solve the problems of lack, targeting of toxic and side effects of drugs, and unclear effectiveness and safety of chronic diseases in vivo.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
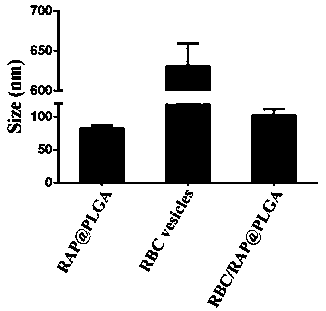
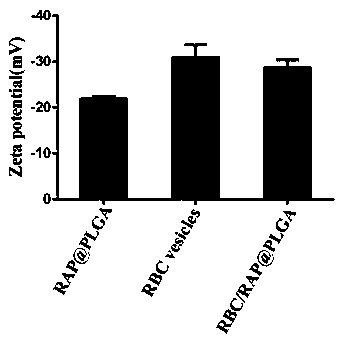
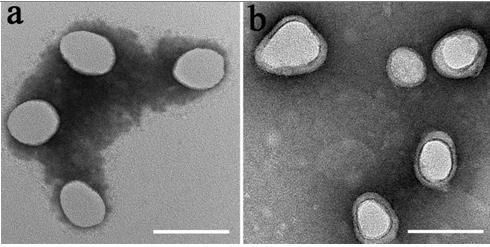
[0038] Example 1: Construction and property characterization of erythrocyte membrane-coated nanomedicine
[0039] ①Preparation of nano-erythrocyte capsule
[0040] The red blood cells were collected from the whole blood through multiple centrifugation, and the nano-red blood cell capsule was prepared by further freeze-thaw cycle technology: a) Red blood cell separation: fresh whole blood was anticoagulated with heparin and ethylenediaminetetraacetic acid (EDTA), and kept at 4°C Centrifuge under the conditions (4000 rpm, 5min), wash with PBS repeatedly 3 times, and collect red blood cells. Red blood cell membranes are prepared by multiple "freeze-thaw cycles". b) Preparation of nano-erythrocyte capsules by freeze-thaw cycles: the erythrocyte suspension was first frozen at -80°C, and then thawed at room temperature. Subsequently, the nano-red blood cell capsule was obtained by centrifugation (14000rpm, 20min) at 4°C, and the PBS solution was washed repeatedly for 3 times. . ...
Embodiment 2
[0056] Example 2: In vitro evaluation of erythrocyte membrane-coated nanomedicine
[0057] ①Characterization of surface membrane proteins of RBC / RAP@PLGA nanoparticles
[0058] In order to confirm that the surface of RBC / RAP@PLGA nanoparticles maintains the original functional membrane proteins, the erythrocyte envelope, nano-erythrocyte envelope, and RBC / RAP@PLGA surface proteins were determined by SDS-PAGE. SDS sample buffer (Invitrogen) was prepared first. They were then run in 4-12% Bis-Tris 10-well mini gels on a BIO-RAD electrophoresis system. The samples were run at 75V for 0.5h and then at 140V for 1h to achieve sufficient protein separation. Finally, polyacrylamide gels were stained with SimplyBlue (Invitrogen) to visualize protein bands.
[0059] ② Protein adsorption
[0060] Serum was used to characterize the protein adsorption properties of nanoparticles. RAP@PLGA and RBC / RAP@PLGA were incubated with serum solutions in PBS (0.01M), respectively, to keep the na...
Embodiment 3
[0077] Example 3: In vivo evaluation of erythrocyte membrane-coated nanomedicine
[0078] ①ApoE - / - mouse AS model
[0079] All animal care and experimental protocols were reviewed and approved. Male C57BL / 6 mice (25-30 g, 8 weeks) were purchased from Peking University Health Science Center (Beijing, China) male apolipoprotein-E deficient (ApoE - / - ) mice. ApoE fed a high-fat diet (HFD, normal diet with 0.5% cholesterol and 5% fat) - / - Mouse model AS.
[0080] ②ApoE - / - mouse AS treatment
[0081] 20 ApoE - / - Mice were randomly divided into 4 groups (5 mice in each group) and fed with HFD for 10 weeks. Mice were treated with RAP@PLGA, RBC / RAP@PLGA, and equivalent RAP (0.7 mg / kg) via the tail vein every three days for 30 days. Inject 5% sucrose as the model control group. During the treatment, the body weight changes of the mice were weighed simultaneously.
[0082] ③ Quantitative analysis of atherosclerotic plaque
[0083] At the end of treatment, ApoE - / - Mice we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com