Application of enoxacin in prevention and/or treatment of flavivirus infection
A technology of enoxacin and flavivirus, which is applied in antiviral agents, medical preparations containing active ingredients, and resistance to vector-borne diseases, etc., to achieve the effect of inhibiting replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
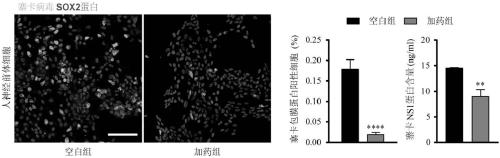
[0034] This example is used to illustrate that enoxacin used in the present invention has an inhibitory effect on the infection of Zika virus on neural precursor cells.
[0035] Treat human neural precursor cells (differentiated from human embryonic stem cells, purchased from Wicll, USA, No. H9, the same below), the concentration of enoxacin in the treatment system was 100 μM, and the concentration of cells was 2×10 5 Individuals / mL, infected with Zika virus (GZ01 strain, GenBank accession number KU820898) after 24 hours, the multiplicity of infection (MOI)=2. After the virus was incubated for 1 hour, replace with 1 mL of new culture solution containing 100 μM enoxacin (purchased from Stemcell, including NeuroCult-XF Proliferation MediuKit, product number 05761; 20 ng / mL EGF, product number 78006; 10 ng / mL bFGF, Product number 78003; 2 μg / mL Heparin, product number 07980, the same below). After 48 hours, the cells were fixed, and the primary antibody of Zika virus envelope p...
Embodiment 2
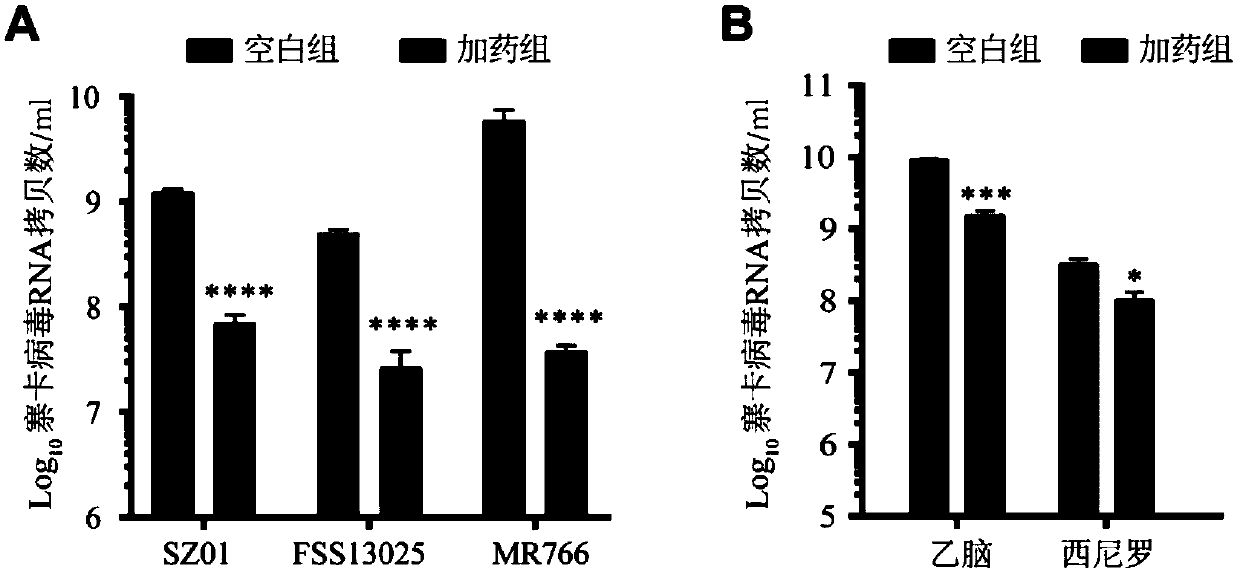
[0037] This example is used to illustrate that enoxacin has an inhibitory effect on the replication of different strains of flaviviruses on neural precursor cells.
[0038] Human neural precursor cells were treated with enoxacin (dissolved in DMSO), the concentration of enoxacin in the treatment system was 100 μM, and the concentration of cells was 10 5Individuals / mL, infected with Zika virus (SZ01, FSS13025, MR766 strain, GenBank accession numbers are KU866423, KU955593 and AY632535 respectively) after 24 hours, MOI=2. After the virus was incubated for 1 hour, replace with 1 mL of new culture solution containing 100 μM enoxacin. After 48 hours, take the virus supernatant, extract RNA (the kit used was purchased from Thermo Fisher Scientific, USA, No. 12183018A, the same below), and detect viral RNA by real-time fluorescent quantitative PCR (see Table 1 for primers, the same below). Copy number, human neural progenitor cells treated with DMSO (without enoxacin) were used as...
Embodiment 3
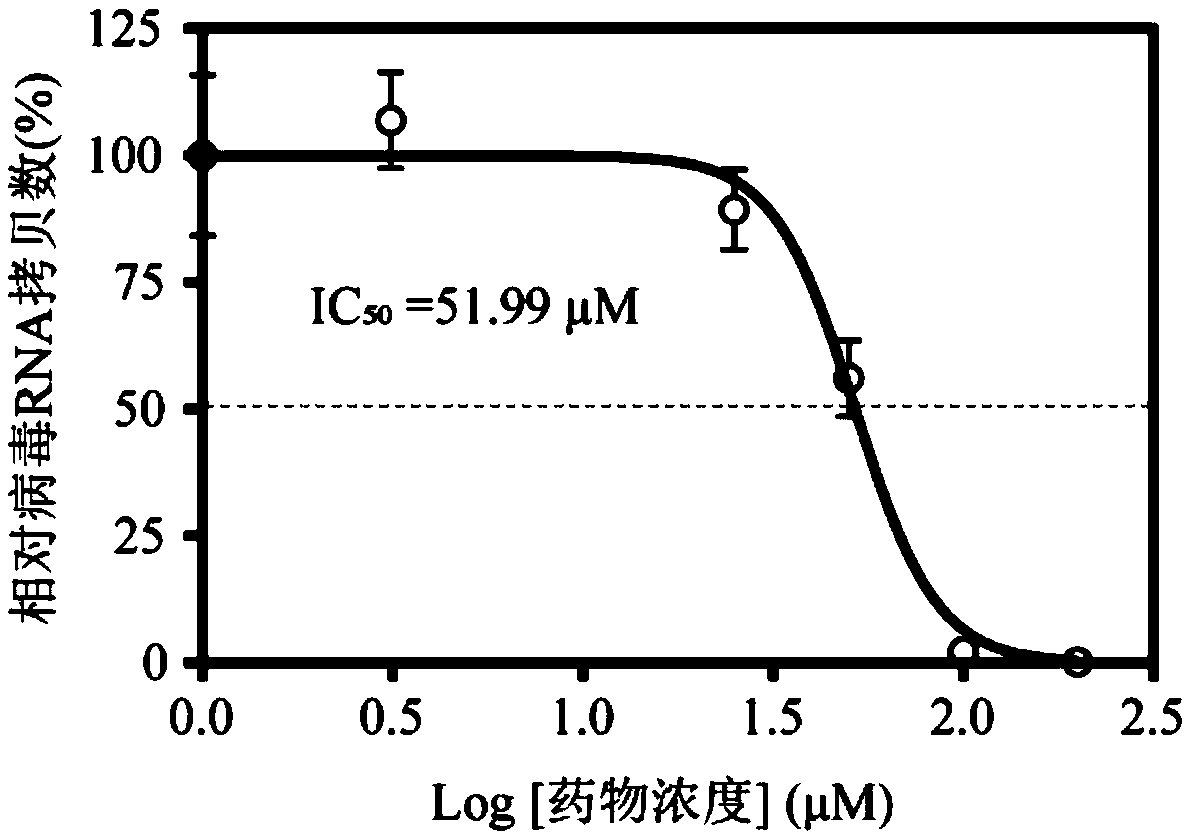
[0044] This embodiment is used to illustrate the IC of enoxacin anti-Zika virus infection 50 Test Results.
[0045] Enoxacin was serially diluted 2 times to make the final use concentration from 200 μM to 3.125 μM. Treat human neural precursor cells, the cell concentration in the treatment system is 2×10 5 individual / mL, infected with Zika virus (GZ01 strain) after 24 hours, MOI=2. After the virus was incubated for 1 hour, 1 mL of culture medium containing gradient concentrations of enoxacin was replaced with new ones. After 48 hours, the virus supernatant was taken to extract RNA, real-time fluorescent quantitative PCR was used to detect the copy number of virus RNA, and the IC was obtained by using GraphPad 7.0 software to process statistics 50 =51.99, see the result image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com