Multilayer pharmaceutically active compound release microparticles in liquid dosage form
A technology for liquid compositions and compounds, applied in the directions of drug combination, drug delivery, organic active ingredients, etc., can solve the problems of time instability, unpleasant taste, unpleasant palatability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
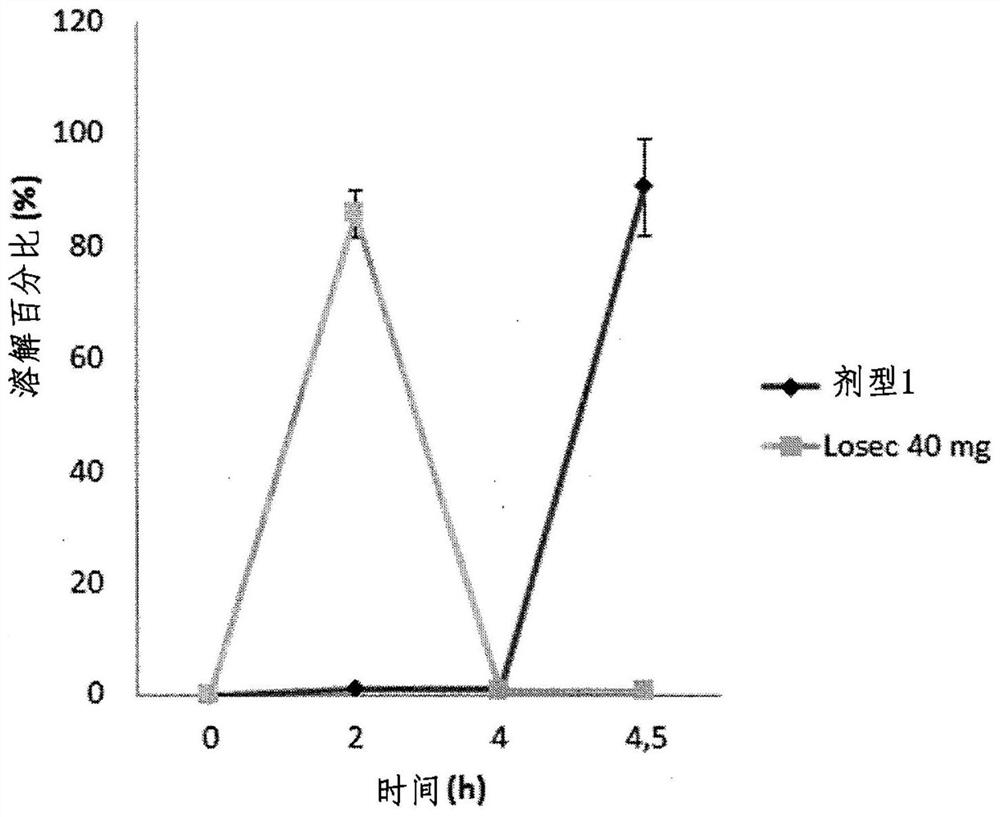
[0201] Example 1: Development of 5-layer coated microparticles according to the invention
[0202] 500g of 1000 poured into the fluidized bed unit.
[0203] The first layer contains: Omeprazole (as model drug, pharmaceutically active compound according to the present invention), ascorbyl palmitate (as antioxidant), PVP (as binder), talc (as bulk agent) )). Disperse the material in ethanol. Therefore, the coating has the first layer of Corresponds to a core comprising a pharmaceutically active compound according to the invention.
[0204] The purpose of the second layer is to combine drugs with L-shaped membrane polymer isolation. This layer contains: PVP (as both release and binding polymer), titanium dioxide (as opacifying agent) and talc (as extender). Disperse the material in ethanol. This second layer corresponds to the protective intermediate coating layer according to the invention.
[0205] The third layer is the effective layer that provides the final relea...
Embodiment 2
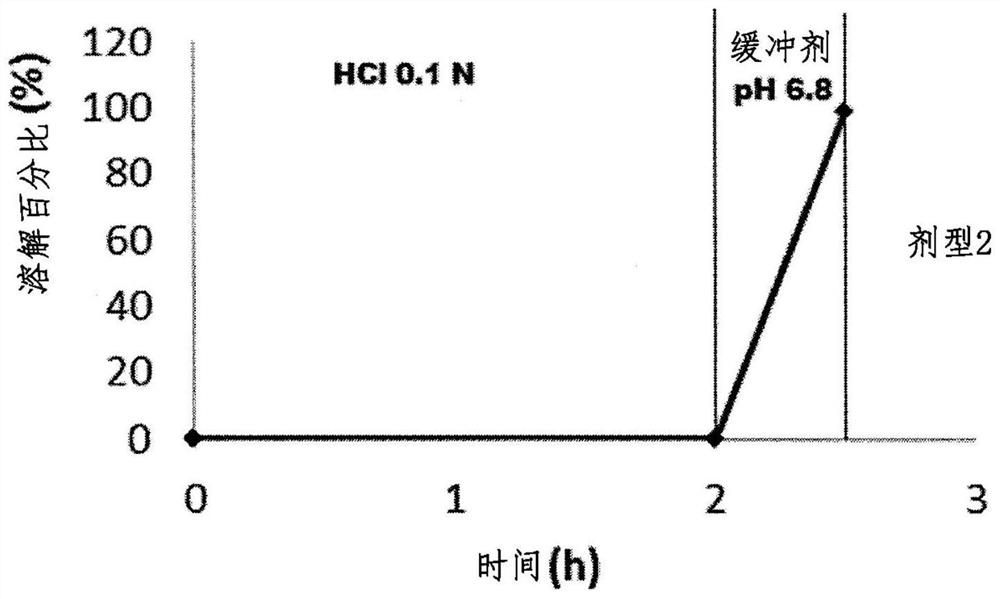
[0218] Example 2: Composition suitable for preparing the outermost outer layer according to the invention
[0219] To test the barrier properties of the outermost outer layer composition, omeprazole-containing microparticles were prepared on which different compositions comprising gastrosoluble polymers and hydrophobic agents were deposited.
[0220] Microparticles were prepared as follows: In the first step, 500 g 1000 (microcrystalline cellulose pellets) were poured into a fluidized bed coater (Aeromatic, STREA-1 TM ; Laboratory scale), and spray the solution / suspension (in ethanol) containing omeprazole, PVP, ascorbyl palmitate with a flow rate of 8g / min with a peristaltic pump. The outermost outer layer is then deposited by spraying a solution comprising: E (polycationic gastrosoluble polymer (a) according to the present invention), and ethyl cellulose, RS, stearic acid, 888 One of ATO, magnesium stearate or GMS (glyceryl monostearate) (as hydrophobic and / or insolu...
Embodiment 3
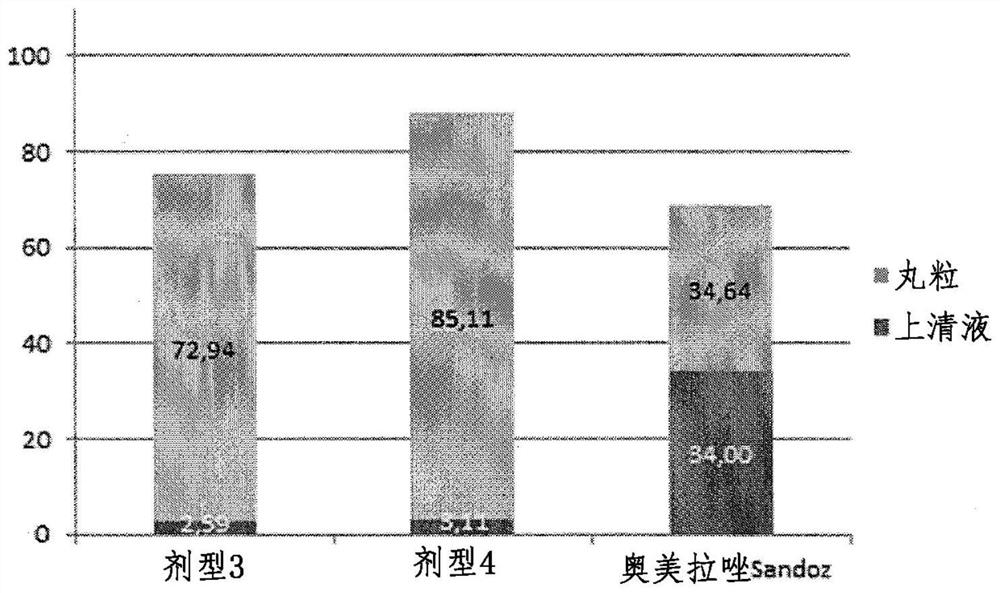
[0238] Example 3: with a GMS and Omeprazole-containing multilayer microparticles of the outermost outer layer of E (Formulation 3 and Form 4)
[0239] Two batches of multilayer microparticles (dosage form 3 and dosage form 4) containing omeprazole were prepared, and the multilayer microparticle coatings were coated with the The outermost outer layer of E and GMS. The samples differ in that in sample formulation 4 there is direct deposition on the microcrystalline cellulose pellets An additional layer of ethylcellulose on the surface to further help limit the diffusion of water from the surrounding aqueous medium to the core of the microparticles.
[0240] preparation:
[0241] The preparation of the core and layer 1 of dosage form 3 was the same as the preparation of the core and the first layer according to example 2 and as described in the coating parameters of materials and methods. In fact, the 500g of 1000 into a fluidized bed apparatus (Aeromatic, Switzerland). ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com