Screening method for serum exosome marker of laryngeal squamous carcinoma and application for exosome source miR-941
A technology of mir-941 and laryngeal squamous cell carcinoma, which is applied in the field of molecular diagnosis and molecular biology, can solve the problems of patients suffering and inability to diagnose early, and achieve reliable diagnostic results, great clinical practical value, and simple and fast clinical diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
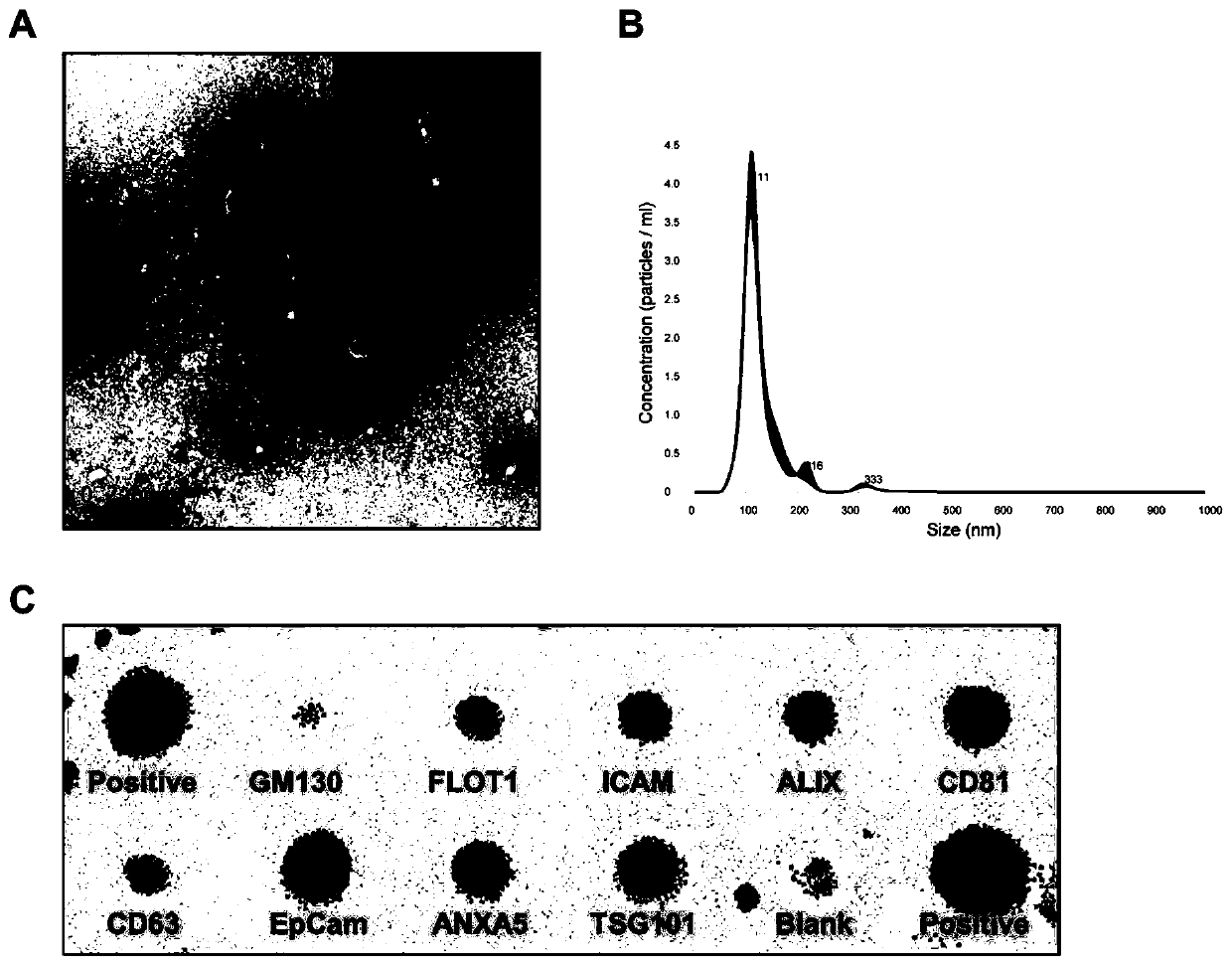
[0032] Serum exosome extraction, identification and exosome RNA extraction:
[0033] Step 1, collection of serum samples
[0034] Collection objects: A total of 87 serum samples were collected, including 56 histopathologically confirmed laryngeal squamous cell carcinoma patients and 31 healthy controls. Patients with squamous cell carcinoma of the larynx were from the Department of Otolaryngology, Head and Neck Surgery, the First Hospital of Shanxi Medical University, and had no history of radiotherapy and chemotherapy or acute and chronic inflammatory diseases, and were used as the experimental group. The healthy control subjects were from the physical examination center of the hospital, had no history of acute and chronic inflammatory diseases and malignant tumors, and matched the age and sex of the experimental group. This study was approved by the Research Ethics Committee of Shanxi Medical University, and all subjects signed informed consent.
[0035] Specific steps: co...
Embodiment 2
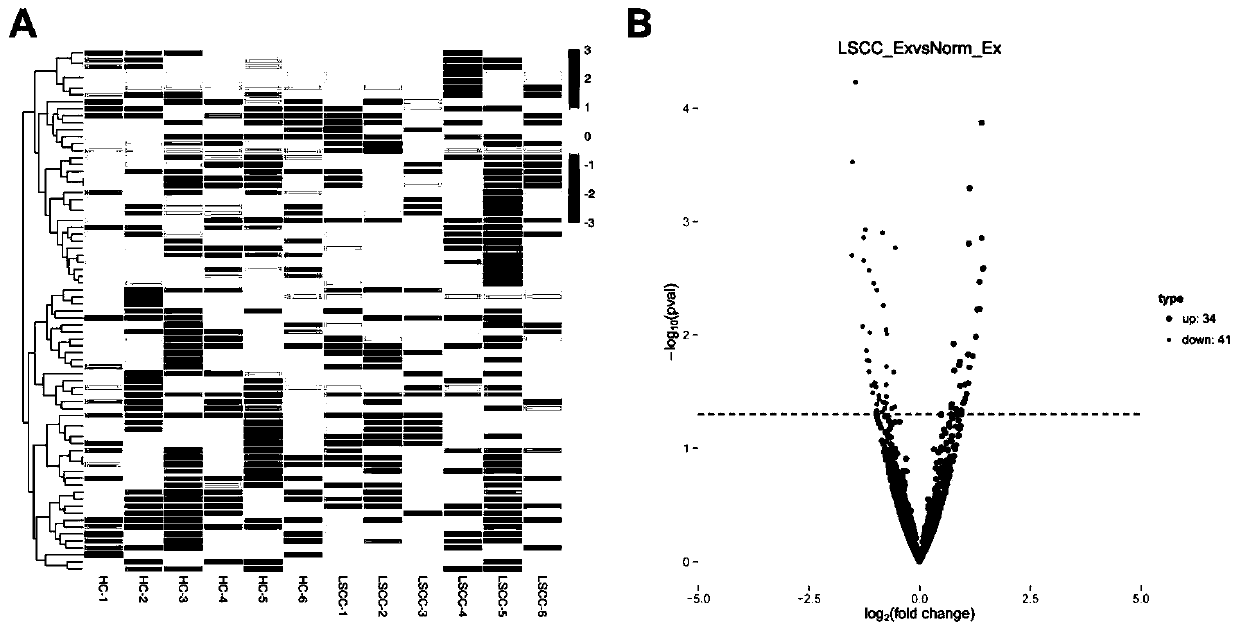
[0045] Screening of differential miRNAs in serum exosomes of patients with laryngeal squamous cell carcinoma and healthy controls by RNA sequencing:
[0046] Serum exosomal RNA from 6 patients with laryngeal squamous cell carcinoma and 6 healthy controls was used for RNA sequencing analysis in this experiment.
[0047] Step 1, sample detection, library construction and sequencing
[0048] (1) Total RNA sample detection: The high-sensitivity Agilent 2100pic600 is used to accurately detect the total amount and fragment distribution of RNA.
[0049] (2) Library construction: After the sample is qualified, use the Small RNA Sample Pre Kit to construct the library, using the special structure of the 3' and 5' ends of Small RNA (the 5' end has a complete phosphate group, and the 3' end has a hydroxyl group), Using total RNA as the starting sample, directly add adapters to both ends of Small RNA, and then reverse transcribe to synthesize cDNA. Subsequently, after PCR amplification,...
Embodiment 3
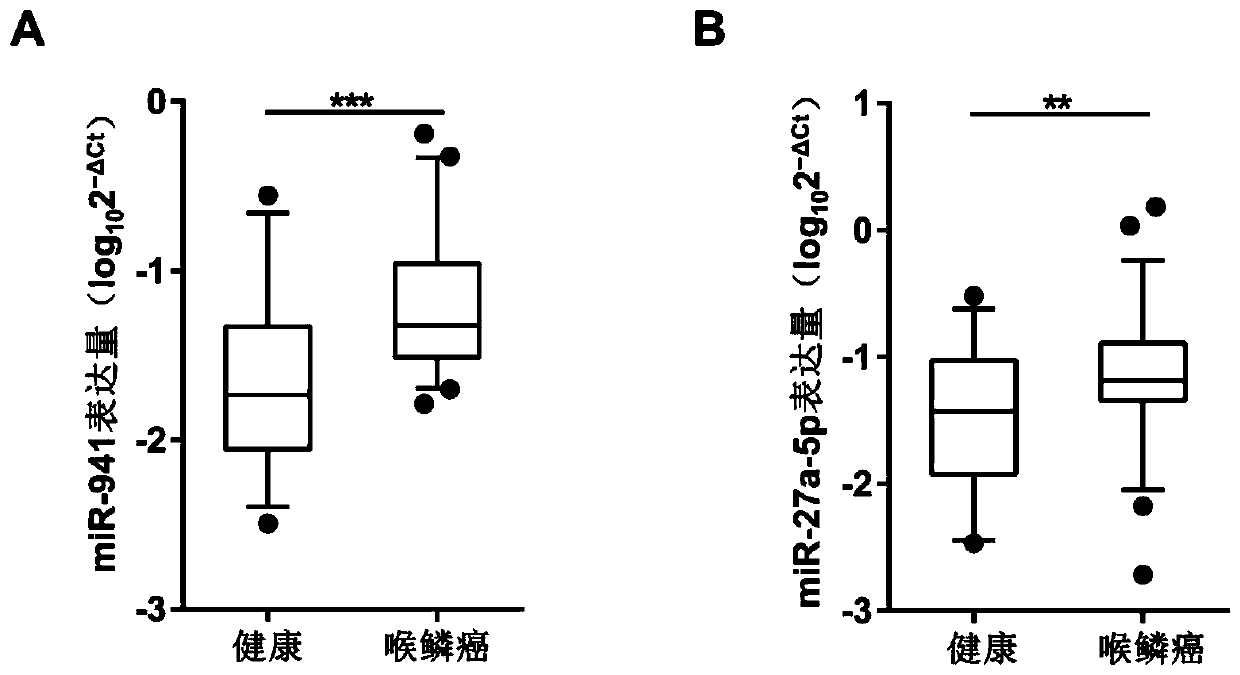
[0055] Real-time fluorescence quantitative (qRT-PCR) verification of serum exosome differences in patients with laryngeal squamous cell carcinoma and healthy controls:
[0056] There is no recognized stable internal reference gene in serum exosomes. In this experiment, the internal reference gene was first screened and identified, and then the qRT-PCR verification of the differentially expressed miRNAs in Experiment 2 was carried out. In this experiment, serum exosomal RNA of 50 patients with laryngeal squamous cell carcinoma and 25 healthy controls was used for qRT-PCR verification of differentially expressed miRNAs, among which, serum exosomal RNA from 7 patients with laryngeal squamous cell carcinoma and 7 healthy controls Body RNA was also used for qRT-PCR detection in internal reference screening. The specific implementation steps are as follows:
[0057] Step 1, internal reference gene screening
[0058] The present invention screens the serum exosomal RNAs stably expr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com