A circRNA biomarker, screening method and diagnostic kit for renal clear cell carcinoma
A clear cell renal cell carcinoma, biomarker technology, applied in biochemical equipment and methods, DNA/RNA fragments, biostatistics, etc., can solve problems such as circRNA biomarkers for renal clear cell carcinoma that have not yet been found, and achieve a good consensus sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
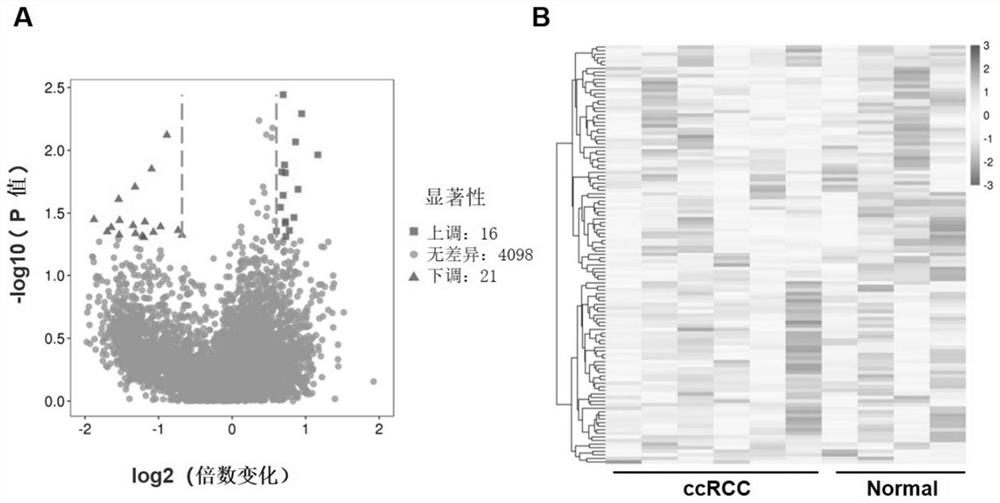
[0045] Example 1 Analysis of circRNA expression profile in renal clear cell carcinoma
[0046] (1) Collection of serum samples from patients with renal clear cell carcinoma
[0047] After obtaining the informed consent of the patients, 6 ml of clear cell renal cell carcinoma patients and 4 healthy people with definite menstrual pathological diagnosis from April 2018 to May 2018 in the First Affiliated Hospital of Zhejiang University in Zhejiang Province were collected (as a control). . All patients with clear cell renal cell carcinoma were newly diagnosed patients and had not undergone surgery, radiotherapy and chemotherapy before blood collection. The 4 healthy controls were age-matched healthy people without malignant tumors and other diseases.
[0048] (2) Serum sample collection and processing
[0049] Draw 6ml of fasting venous blood in the morning and put it in a tube without anticoagulant, let it stand for 30 minutes, centrifuge at 3,000g for 15 minutes at 4°C, take ...
Embodiment 2
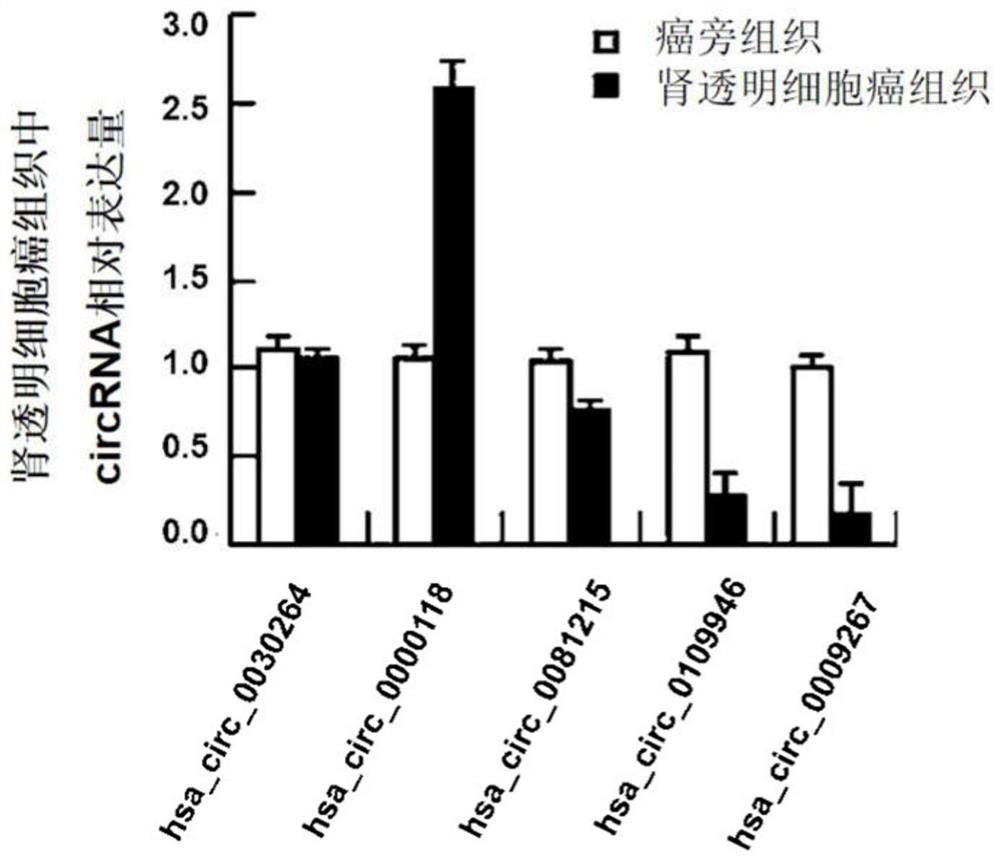
[0052] Example 2 Preliminary screening of target circRNA
[0053] (1) Collect tissue samples from patients with renal clear cell carcinoma
[0054] After obtaining informed consent from patients, 6 pairs of postoperative specimens from patients with pathologically confirmed clear cell renal cell carcinoma from April 2018 to May 2018 in the First Affiliated Hospital of Zhejiang University, Zhejiang Province were collected, including cancer tissue specimens and paired distance cancer tissue 3 All patients have not received chemotherapy and radiotherapy before surgery. All tissue samples were packed in RNase-free EP tubes and stored in a -80°C freezer.
[0055] 2. Extraction of total RNA from tissue samples
[0056] Specific steps are as follows:
[0057] (1) Weigh the tissue cut pieces with a weight between 0.3-0.5g, add 1ml of AG RNAex Pro Reagent (Aikeray Bioengineering Co., Ltd.) lysate, and add magnetic beads, use a tissue disruptor to disrupt the tissue, and homogenize t...
Embodiment 3
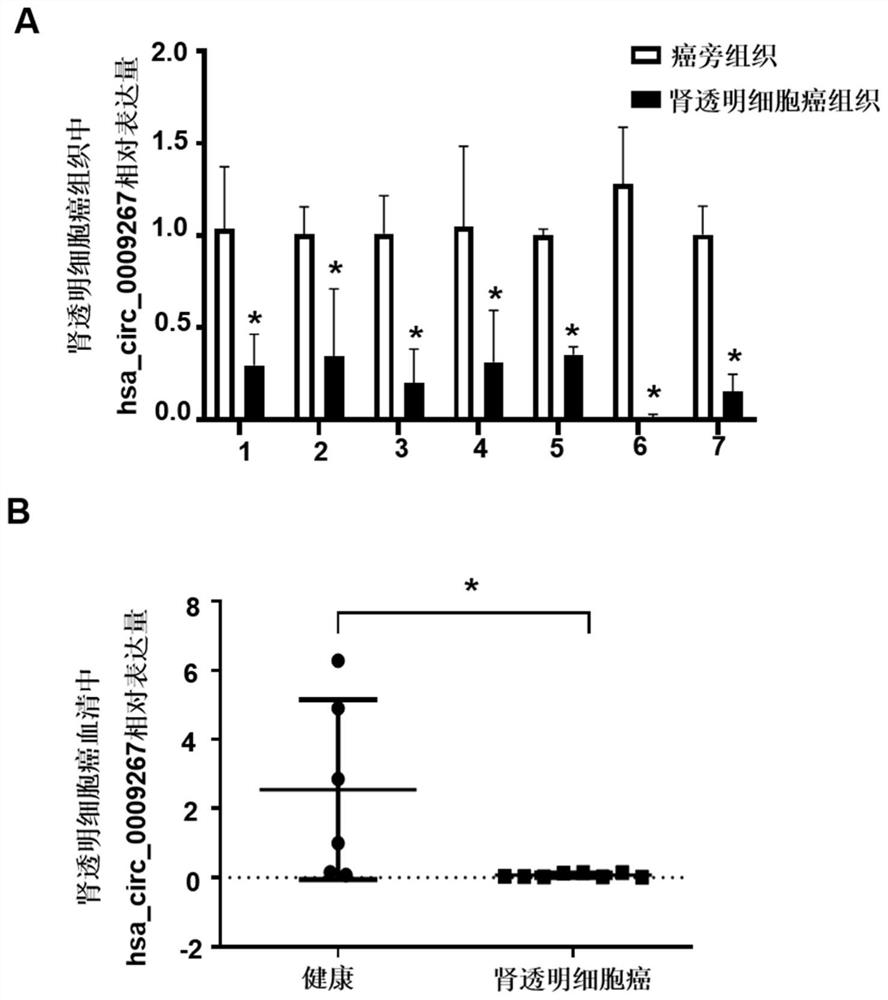
[0087] Example 3 Clinical small sample verification of target circRNA
[0088] 1. Collection of clinical samples
[0089] The present invention selects 7 pairs of renal clear cell carcinoma and adjacent tissue samples, they are respectively from 7 renal clear cell carcinoma patients, selects the serum of 6 healthy controls and 8 renal clear cell carcinoma patients, and the renal clear cell carcinoma tissue samples. The collection and preservation of samples were the same as those in Example 2, and the collection and preservation of serum samples were the same as those in Example 1.
[0090] 2. Extraction of total RNA
[0091] The extraction of total RNA from renal clear cell carcinoma tissue samples was the same as that in Example 2.
[0092] The extraction steps of total RNA from serum samples are as follows:
[0093] (1) Prepare 500 μl of thawed serum samples, add 2500 μl of AG RNAex Pro Reagent lysis reagent, mix well on a vortex shaker or invert several times, and let s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com