Patents
Literature
63 results about "Clear cell carcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Clear-cell carcinoma also known as clear cell adenocarcinoma and mesonephroma is an epithelial cell derived carcinoma characterized by the presence of clear cells observed during histological, diagnostic assessment. This form of cancer is classified as a rare cancer with an incidence of 4.8% in white patients, 3.1% in black patients, and 11.1% in Asian patients.
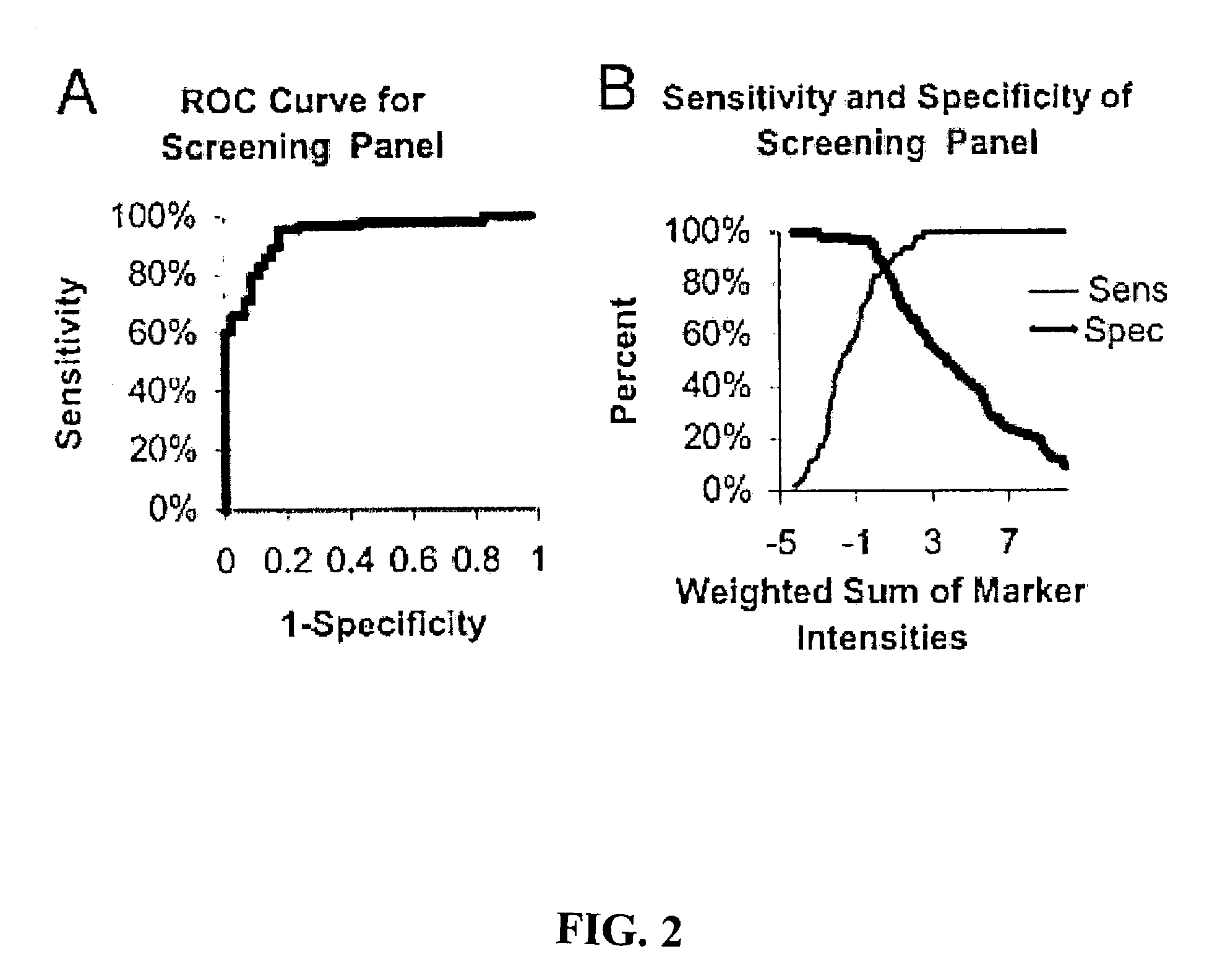
Novel biomarkers and targets for ovarian carcinoma
InactiveCN103026227AOrganic active ingredientsMicrobiological testing/measurementUterine carcinomaARID1A
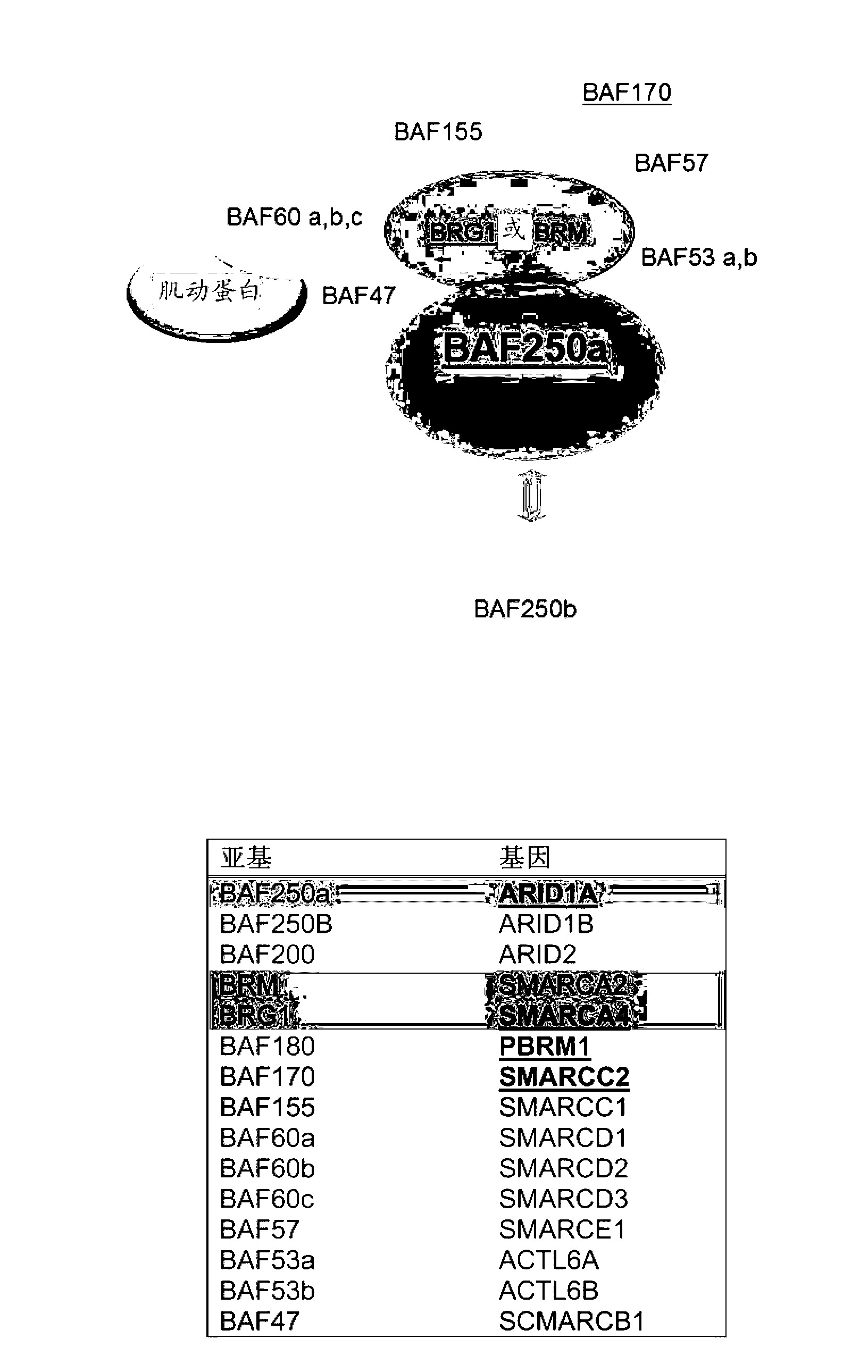
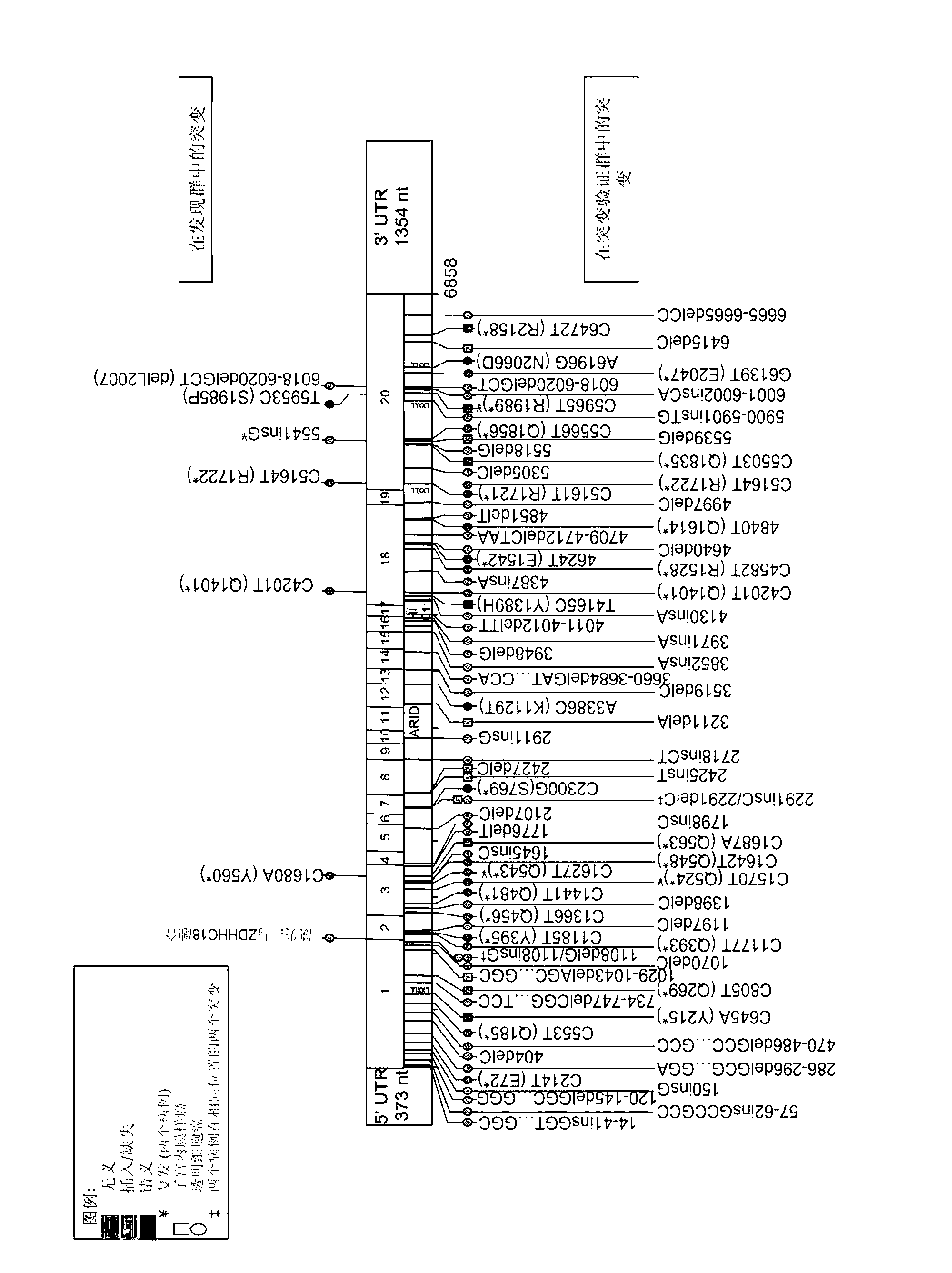
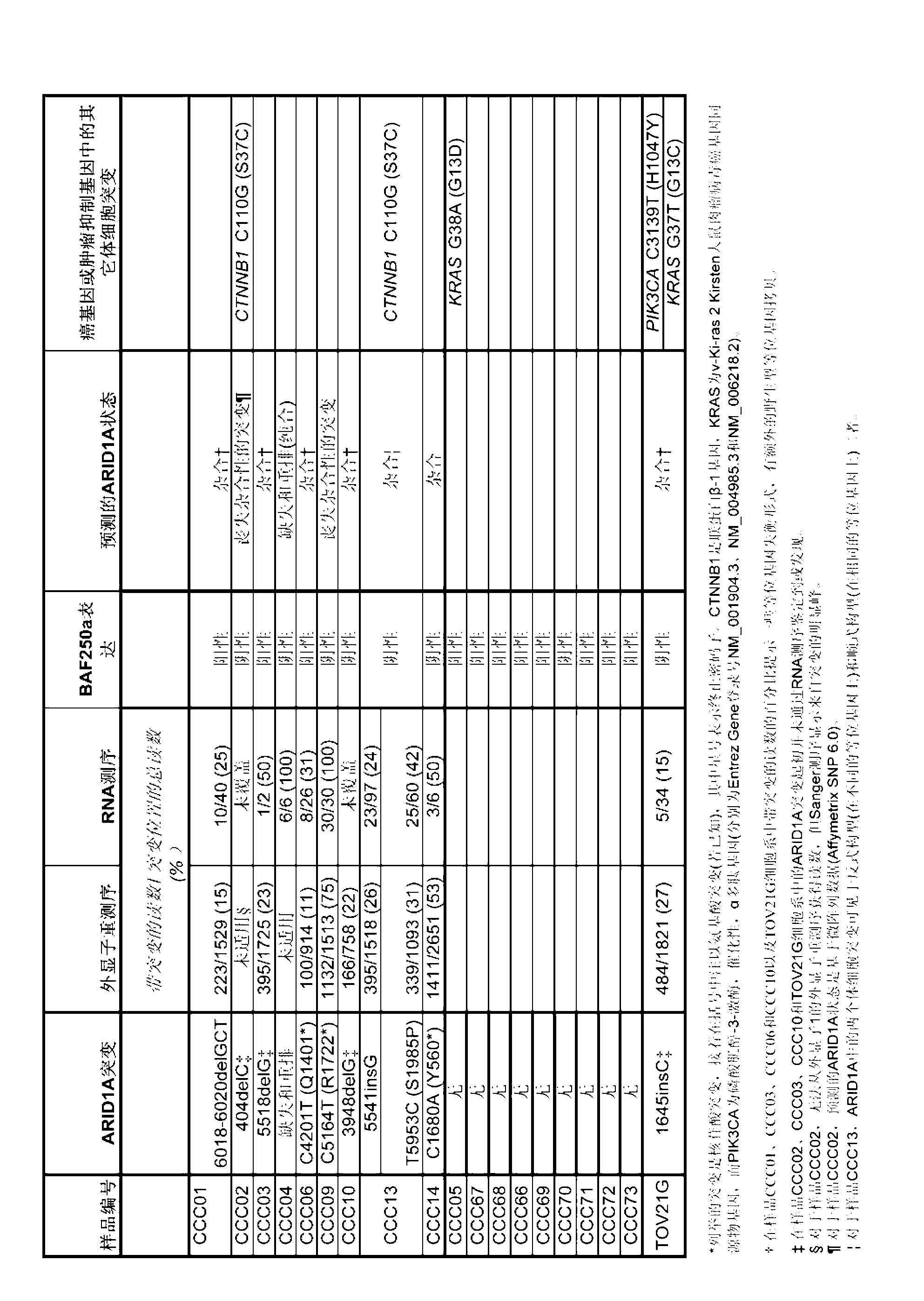
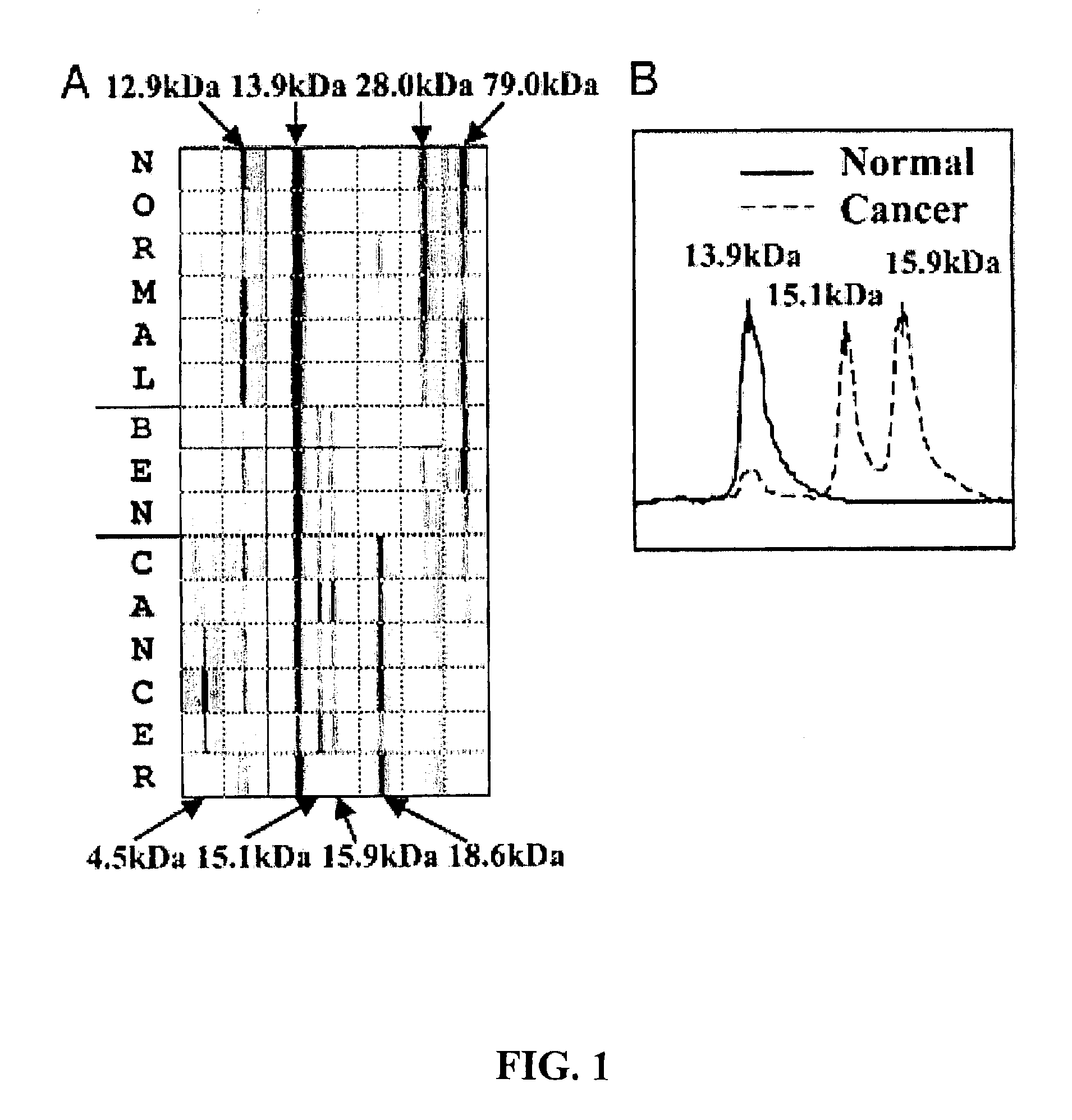
Novel biomarkers and targets associated with ovarian cancer, particularly clear-cell carcinoma, endometrioid carcinoma, and uterine carcinoma, are disclosed. Mutations in genes encoding proteins that form part of the SWI / SNF chromatin remodelling protein complex, including ARID1A, or loss of expression of such proteins, including BAF250a, can be used to evaluate the likelihood endometriosis will progress or transform to cancer, to provide a prognosis for a patient with cancer, to assess whether conventional treatment is likely to be effective against a cancer, and / or in a synthetic lethal screen to identify novel targets and therapeutics for the treatment of cancer.
Owner:BRITISH COLUMBIA CANCER AGENCY BRANCH
Biomarkers for detection of early- and late-stage endometrial cancer
InactiveUS20110027894A1Prevent proliferationBiological material analysisBiological testingLate stageEndometrial cancer
Biomarker proteins that can be used in the diagnosis of early-stage endometrial cancer are described. Methods of screening for endometrial cancer, as well as methods of detecting and monitoring the status of endometrial cancer are provided. The biomarkers can be used to detect a variety of endometrial cancers, including endometroid carcinoma, clear cell carcinoma and serous carcinoma.
Owner:RGT UNIV OF CALIFORNIA
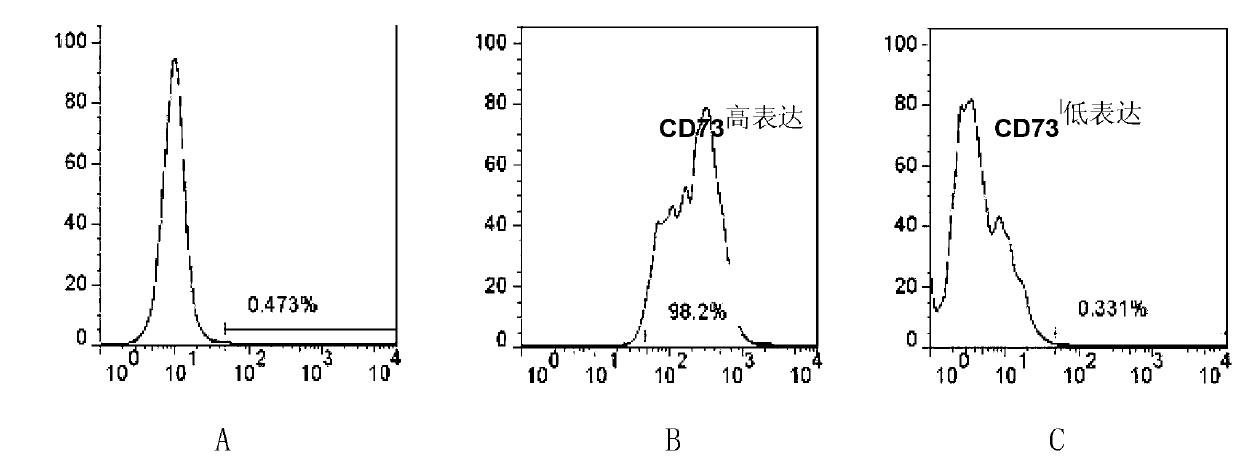
Application of CD73 as stem cell surface marker of renal clear cell carcinoma
InactiveCN103278634ASimple methodAntibody ingredientsUrinary disorderSurface markerKidney Clear Cell Carcinoma
The invention discloses an application of CD73 as a stem cell surface marker of renal clear cell carcinoma. The application is that a substance for detecting CD73 can be used for preparing the following products: 1) a product for detecting or screening the stem cells of renal clear cell carcinoma; 2) a product for diagnosis or auxiliary diagnosis of renal clear cell carcinoma; 3) a product for diagnosis or auxiliary diagnosis of the malignancy degree of renal clear cell carcinoma of a patient to be detected; and 4) a product for treating renal clear cell carcinoma by taking CD73 as a target. In-vitro experiments and animal experiments prove that the CD73 can be used as a surface marker of the stem cells of renal clear cell carcinoma; and the result of clinical sample analysis indicates that the proportion of CD73 positive cells is closely related to the clinical classification of renal clear cell carcinoma. The invention provides a new molecular target CD73 to the identification of the stem cells of renal clear cell carcinoma as well as the diagnosis, classification and detection of renal clear cell carcinoma, and provides a simple and efficient method for the prognosis of renal clear cell carcinoma.
Owner:INST OF MODERN PHYSICS CHINESE ACADEMY OF SCI
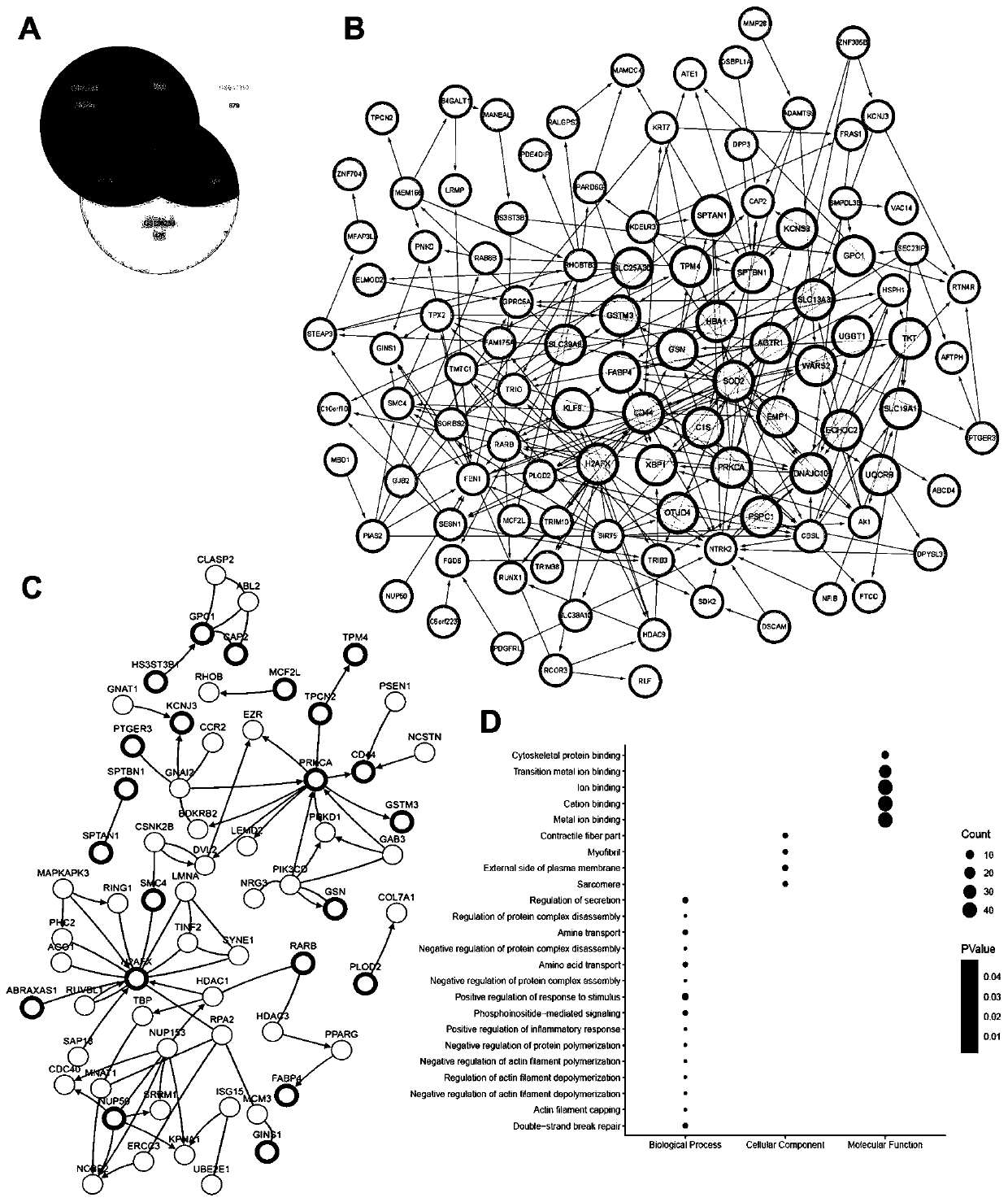
Combined genome for evaluating prognosis of clear cell renal cell carcinoma (ccRCC) and application of combined genome
PendingCN111575376AImprove survival rateMicrobiological testing/measurementDNA/RNA fragmentationRenal clear cell carcinomaClear cell renal cell carcinoma
The invention discloses a combined genome for evaluating prognosis of clear cell renal cell carcinoma (ccRCC) and application of the combined genome, relates to the technical field of medical biological detection, and provides novel application of the combined genome of ADAMTS9, C1S, DPYSL3, H2AFX, MINA, PLOD2, RUNX1, SLC19A1, TPX2 and TRIB3, particularly application in preparation of a ccRCC prognosis evaluation reagent or kit. The genome is derived from a molecular marker significantly correlated with a ccRCC metastasis way, and the discovery of the genome model provides a new strategy for predicting the ccRCC recurrent risk and the long-term survival condition of a patient after operation, plays important roles in judging the prognosis of the ccRCC patient, can evaluate the risk level of tumor progression or death of the patient after ccRCC operation, contributes to guiding a clinician to perform an individualized precise therapeutic strategy, can increase the postoperative survivalrate of the patient, and has important guide significance postoperative follow-up monitoring and sequential therapy management of the ccRCC patient.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
A group of genes for molecular subtyping of renal cell carcinoma and application of genes
ActiveCN107723368AImprove survival ratePrecision medicineMicrobiological testing/measurementDNA/RNA fragmentationFOSBSERPINA5 gene
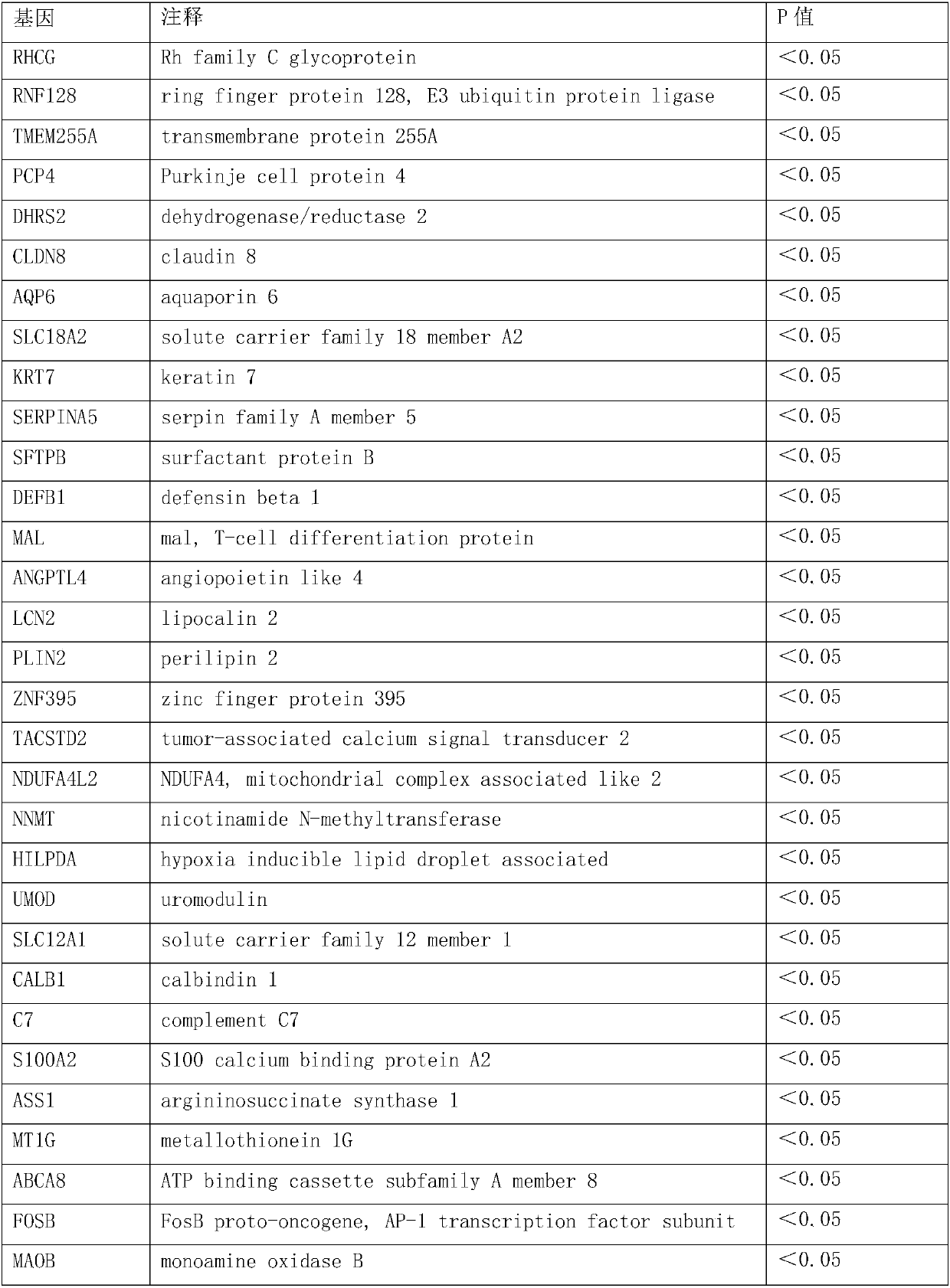
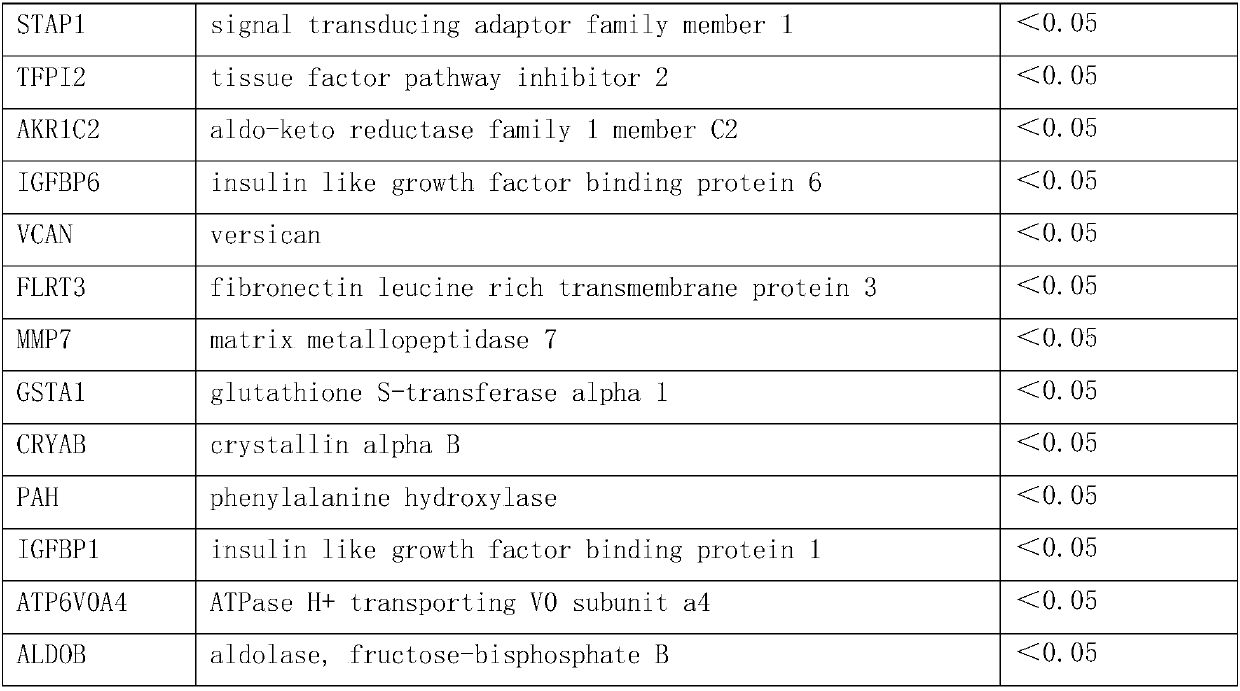
The invention discloses a group of genes for molecular subtyping of renal cell carcinoma and an application of the genes. The group of genes include 44 genes as follows: RHCG gene, RNF128 gene, TMEM255A gene, PCP4 gene, DHRS2 gene, CLDN8 gene, AQP6 gene, SLC18A2 gene, KRT7 gene, SERPINA5 gene, SFTPB gene, DEFB1 gene, MAL gene, ANGPTL4 gene, LCN2 gene, PLIN2 gene, ZNF395 gene, TACSTD2 gene, NDUFA4L2 gene, NNMT gene, HILPDA gene, UMOD gene, SLC12A1 gene, CALB1 gene, C7 gene, S100A2 gene, ASS1 gene, MT1G gene, ABCA8 gene, FOSB gene, MAOB gene, STAP1 gene, TFPI2 gene, AKR1C2 gene, IGFBP6 gene, VCAN gene, FLRT3 gene, MMP7 gene, GSTA1 gene, CRYAB gene, PAH gene, IGFBP1 gene, ATP6V0A4 gene and ALDOB gene. Based upon verification, it is proved that the genes can be used for accurately distinguishing oncocytic adenom, clear cell carcinoma, papillary cell carcinoma chromophobe renal carcinoma; the genes are broad in application scope, high in accuracy rate and short in experimental cycle; and the genes can achieve an important clinical significance for the precise treatment of a patient.
Owner:HANGZHOU CANHELP GENOMICS TECH CO LTD
Diagnosis marker and treatment target of renal clear cell carcinoma
InactiveCN107385083ATreatment reachedCompound screeningApoptosis detectionLymphatic SpreadCancer cell
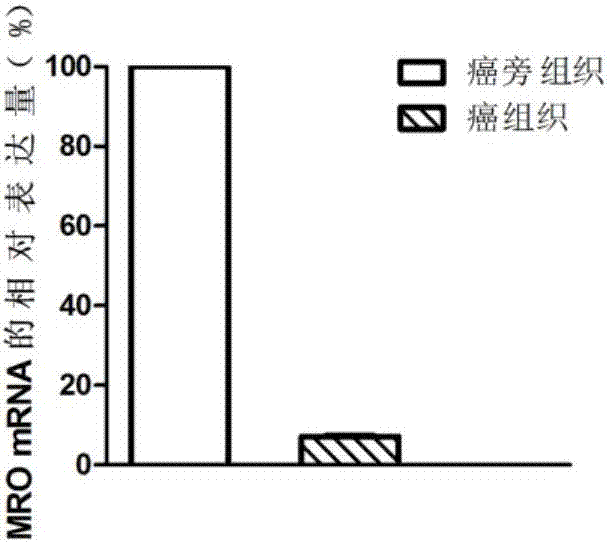
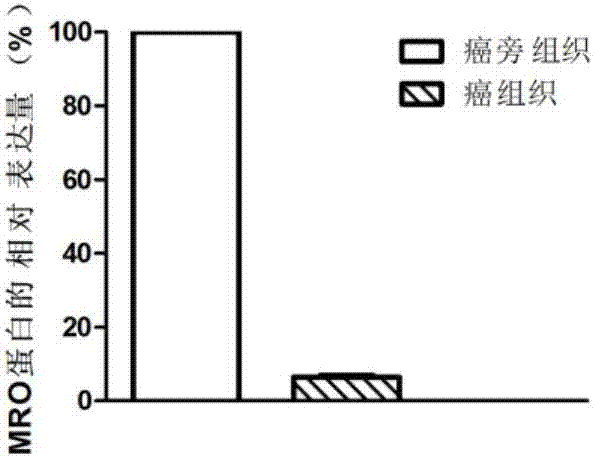
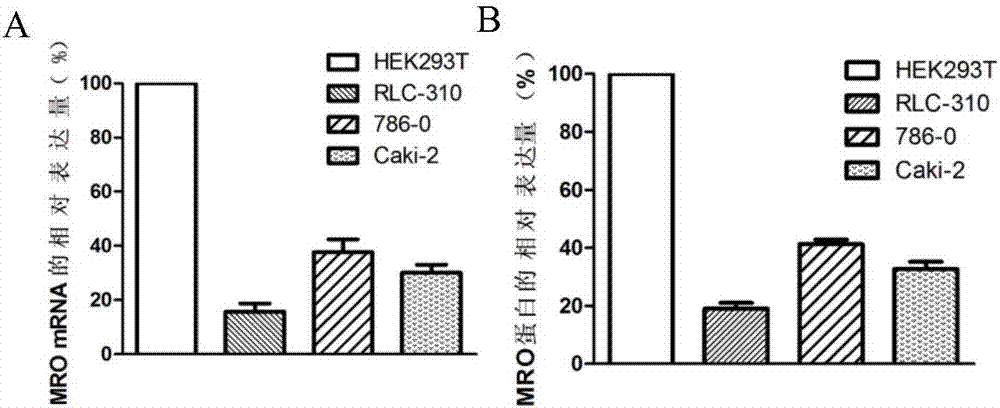
The invention discloses a diagnosis marker and treatment target of renal clear cell carcinoma and belongs to the technical field of gene new application. The diagnosis marker and treatment target is MRO. The diagnosis marker and treatment target has the advantages that the fact that the expression of the marker MRO in a renal clear cell carcinoma is reduced is discovered for the first time, the proliferation, migration and invasion of cancer cells can be lowered by increasing the expression of the MRO, and the MRO serving as the target can be used for treating the renal clear cell carcinoma and renal carcinoma metastasis.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Cell line for lowly-metastatic clear cell carcinoma of kidney of Han Chinese
InactiveCN101550407AMicrobiological testing/measurementMicroorganism based processesCancer cellKidney
The invention discloses a cell line for lowly-metastatic clear cell carcinoma of kidney of Han Chinese, namely LoMeT-ccRcc CCTCC No.C200910. The cell line can grow for a long time in vitro and be stably handed down, has epithelioid cell form, and has no contact inhibition. The cell is heteroploid, chromosome number and structure are aberrant. Immunohistochemistry shows masculine CA9 and CD133. A flow cytometry analyses and finds out that CA9 and CD133 expression is obviously enhanced in the condition that cell is handed down for many times. The cell line has lowly-metastatic trend after the cell is handed down continuously. The recovery rate of the cell line after being frozen is more than 80%, the recovered cell growth state accords with the original cell. The cell line of the invention is used for establishing a cell model for sieving and preparing medicines that is used for dialogising, preventing and treating clear cell carcinoma of kidney.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Biomarker relating to renal clear cell carcinoma
ActiveCN107496923APrecision therapyAchieve early detectionOrganic active ingredientsGenetic material ingredientsCell cancerCancer cell
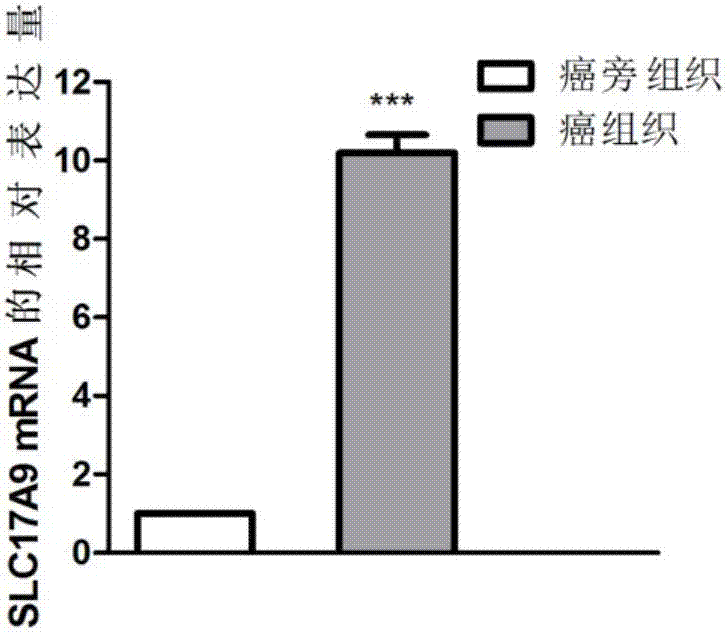
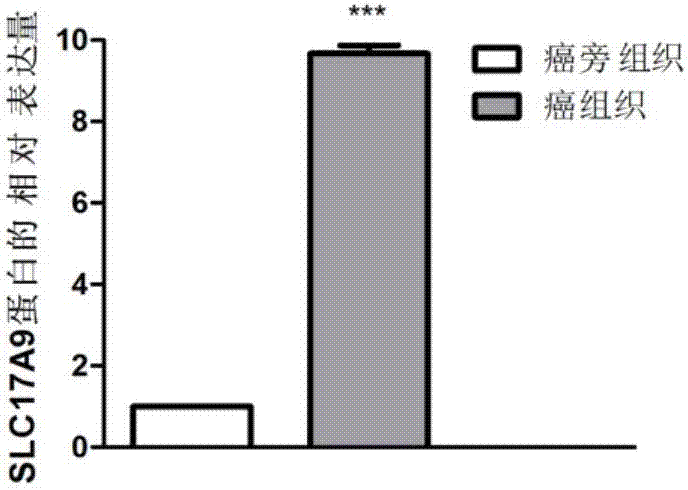
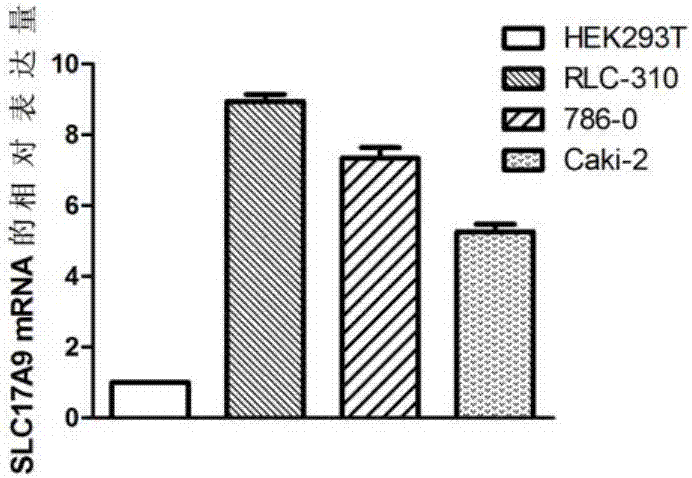
The invention discloses a biomarker relating to renal clear cell carcinoma. The biomarker is SLC17A9. Experiments prove that the expression of the SLC17A9 is up-regulated in patients suffering from the renal clear cell carcinoma, proliferation and invasion of the renal clear cell carcinoma cells can be inhibited, and the SLC17A9 can serve as a diagnosis and / or treatment target to be applied to clinical renal clear cell carcinoma diagnosis and treatment.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Application of lncRNA combination to preparation of product for predicting renal clear cell carcinoma prognosis and molecular targeted medicine treatment sensitivity
InactiveCN108277283ASensitivity PreciseSensitivity Rapid JudgmentMicrobiological testing/measurementMolecular targeted drugRenal clear cell carcinoma
The invention discloses application of lncRNA combination to preparation of a product for predicting renal clear cell carcinoma prognosis and molecular targeted medicine treatment sensitivity. The lncRNA or the lncRNA combination serving as a detection target point is applied to preparation of the product for predicting renal clear cell carcinoma prognosis and molecular targeted medicine treatmentsensitivity, and the lncRNA or the lncRNA combination is selected from any one or more of SEQ ID NO.1 to SEQ ID NO.4. A new clinical method is provided for evaluating prognosis of patients sufferingfrom renal clear cell carcinoma, new reference advice is provided for auxiliary treatment after operation of the renal clear cell carcinoma, and important clinical significance and popularization andapplication prospect in the aspects of timely taking effective clinical measures, formulating an individualized diagnosis and treatment scheme and finally increasing the survival rate of the patientssuffering from the renal clear cell carcinoma are achieved.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
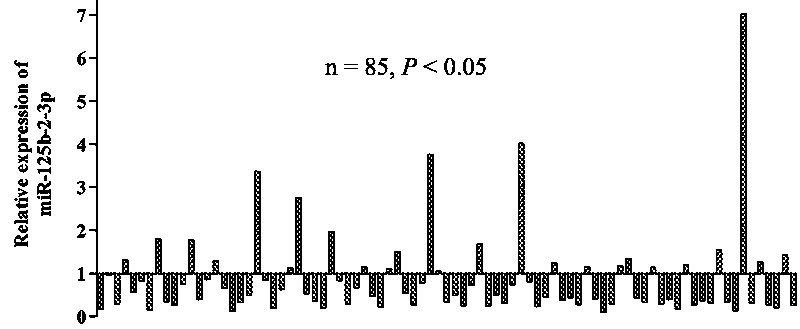
MiR-125b-2-3p as molecular marker for differential diagnosis of renal cancer subtypes and use thereof in tumor metastasis
The present invention relates to a MiR-125b-2-3p as a molecular marker for differential diagnosis of renal cancer subtypes and a use thereof in tumor metastasis. The biomolecular marker MiR-125b-2-3prelated to genesis and development of renal clear cell carcinoma is firstly discovered. By detecting an expression of the MiR-125b-2-3p in patient tissues and blood, early diagnosis of the renal clearcell carcinoma is experimented. Besides, a molecular target for treatment of the renal clear cell carcinoma is firstly provided. The treatment by the targeting and molecular marker in the metastaticrenal clear cell carcinoma has sensitivity and specificity.
Owner:NINGBO UNIV
Application of miR-577 for preparing nephrosis diagnosis marker
InactiveCN108624693AMicrobiological testing/measurementAntineoplastic agentsNephrosisTarget analysis
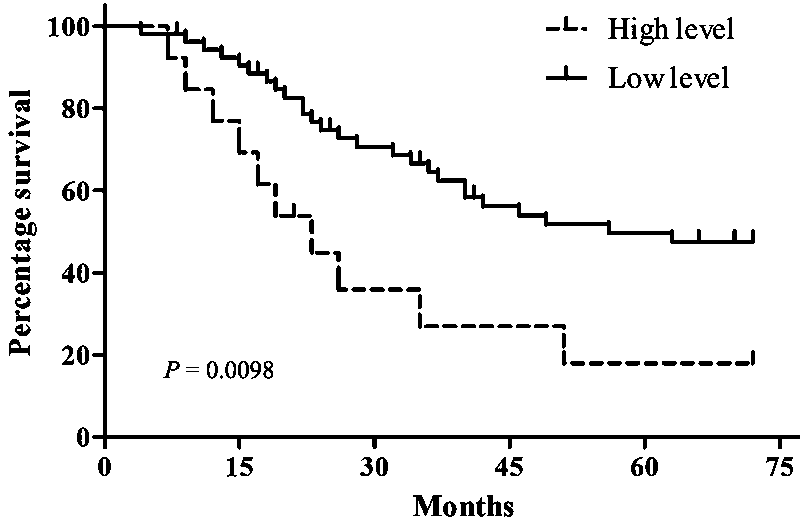
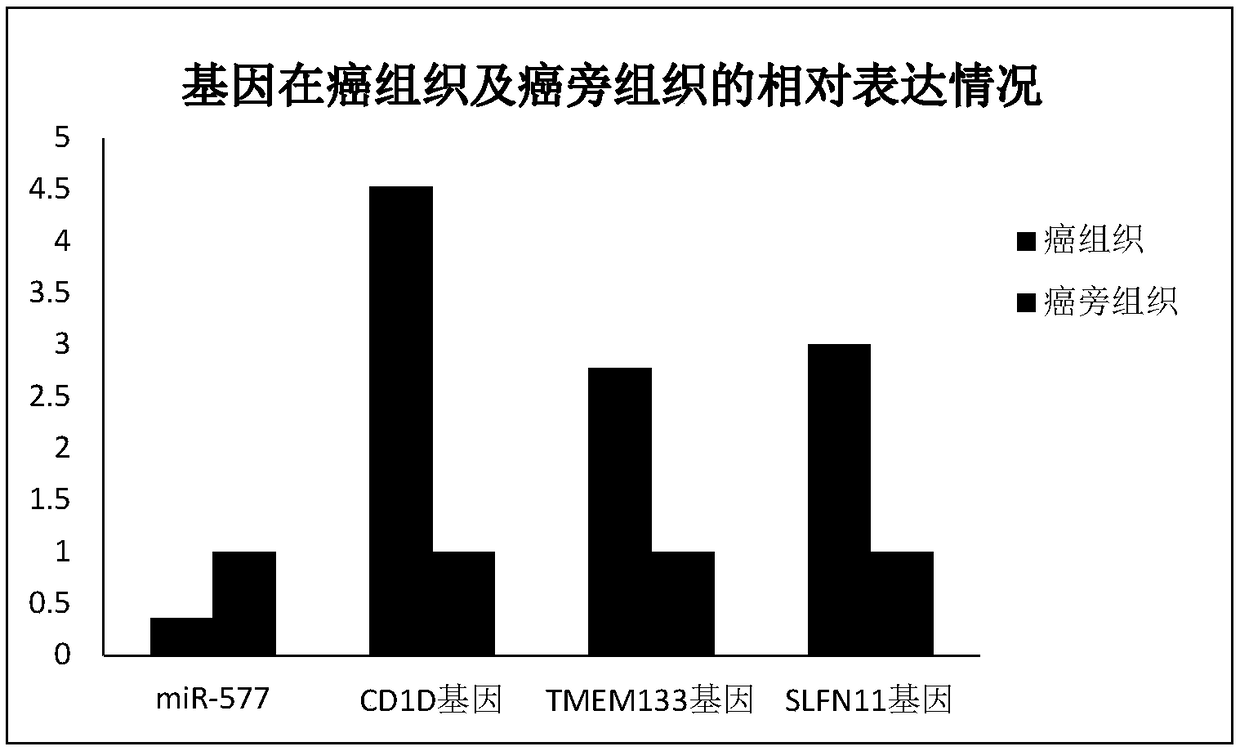
The invention relates to application of miR-577 for preparing a nephrosis diagnosis marker, in particular to application of miR-577 and a target gene thereof in preparing a renal clear cell carcinomadiagnosis marker. Database renal clear cell carcinoma mRNA (Ribonucleic Acid) and miRNA data are retrieved, and miR-577 and a target gene regulated and controlled by the miR-577 are selected to carryout molecular verification through data integration, differential expression analysis and targeted analysis. A result indicates that the miR-577 and the target genes CD1D, TMEM133 and SLFN11 regulatedand controlled by the miR-577 can serve as the diagnosis marker of the renal clear cell carcinoma, and therefore good clinic application value is performed.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Aptamer for detecting human renal clear cell carcinoma cells and application of aptamer in preparing detection preparation
ActiveCN106754938AHigh affinityImprove featuresMaterial analysisDNA/RNA fragmentationAptamerCancer cell
The invention discloses an aptamer for detecting human renal clear cell carcinoma cells (786-O) and application of the aptamer in a preparing detection preparation. Compared with the prior art, the aptamer has affinity and specificity higher than those of an associated protein antibody, is free of immunogenicity, can be chemically synthesized in vitro and is small in molecular weight. Furthermore, different parts of the aptamer can be modified and replaced, so that sequences are stable, storage is convenient, and labeling is convenient. When the aptamer disclosed by the invention is adopted to detect the human renal clear cell carcinoma cells, operations are simpler and quicker; and moreover, synthesis cost of the aptamer is lower than preparation cost of the antibody, the period is short and repeatability is good.
Owner:HUNAN UNIV
Application of novel molecular marker to preparation of kit for diagnosis and prognosis of renal clear cell carcinoma
ActiveCN108559777AHigh sensitivityStrong specificityMicrobiological testing/measurementProteomicsMathematical modelTumor Sample
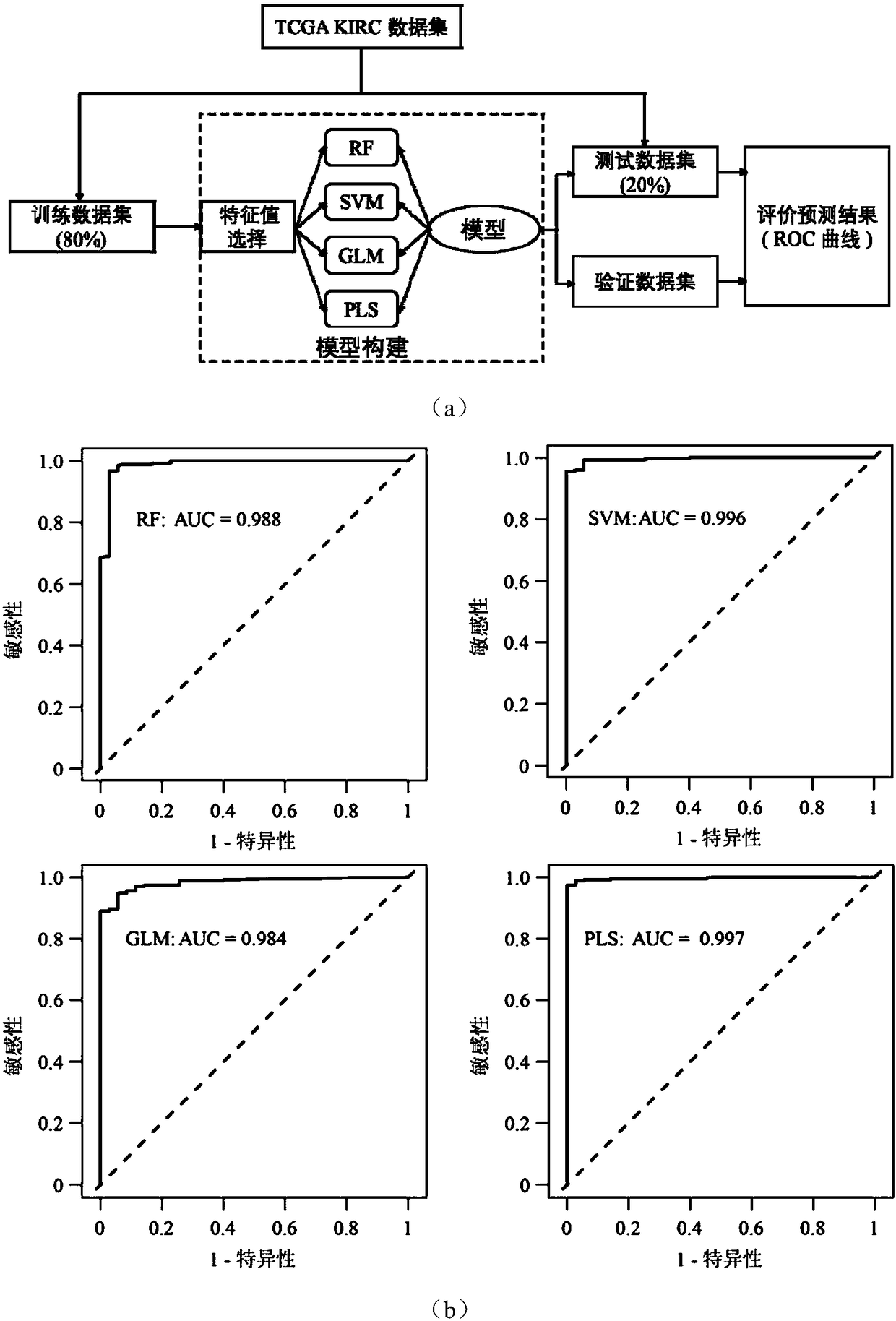
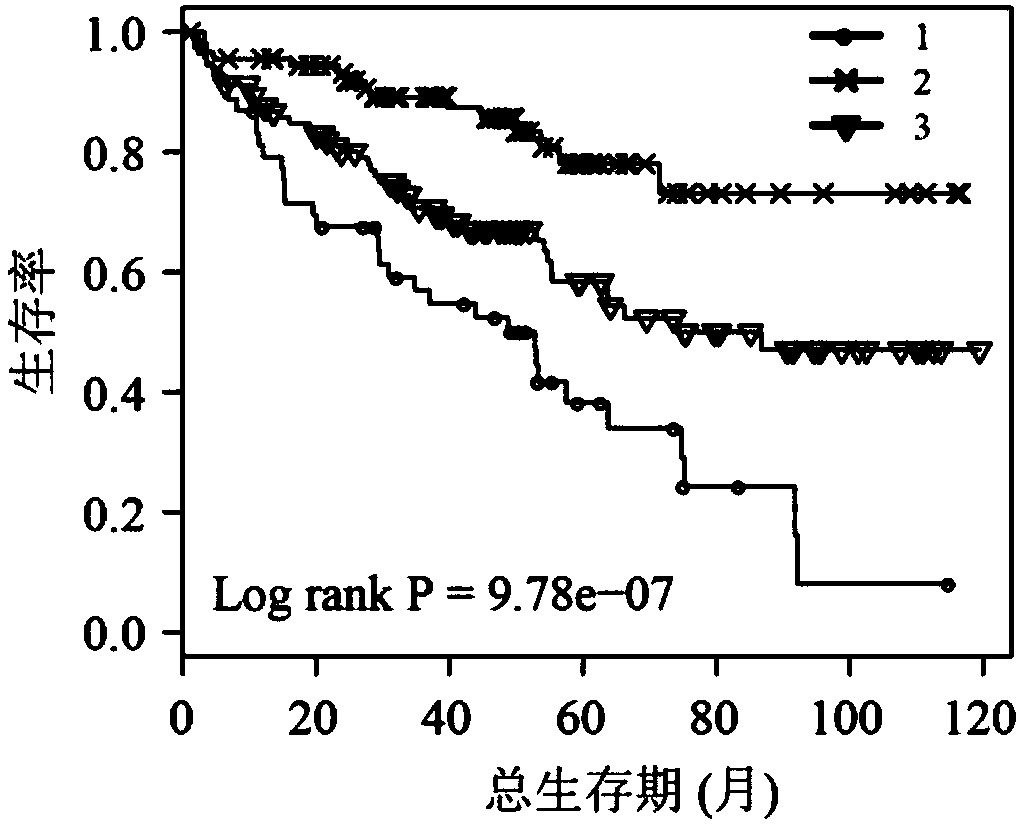
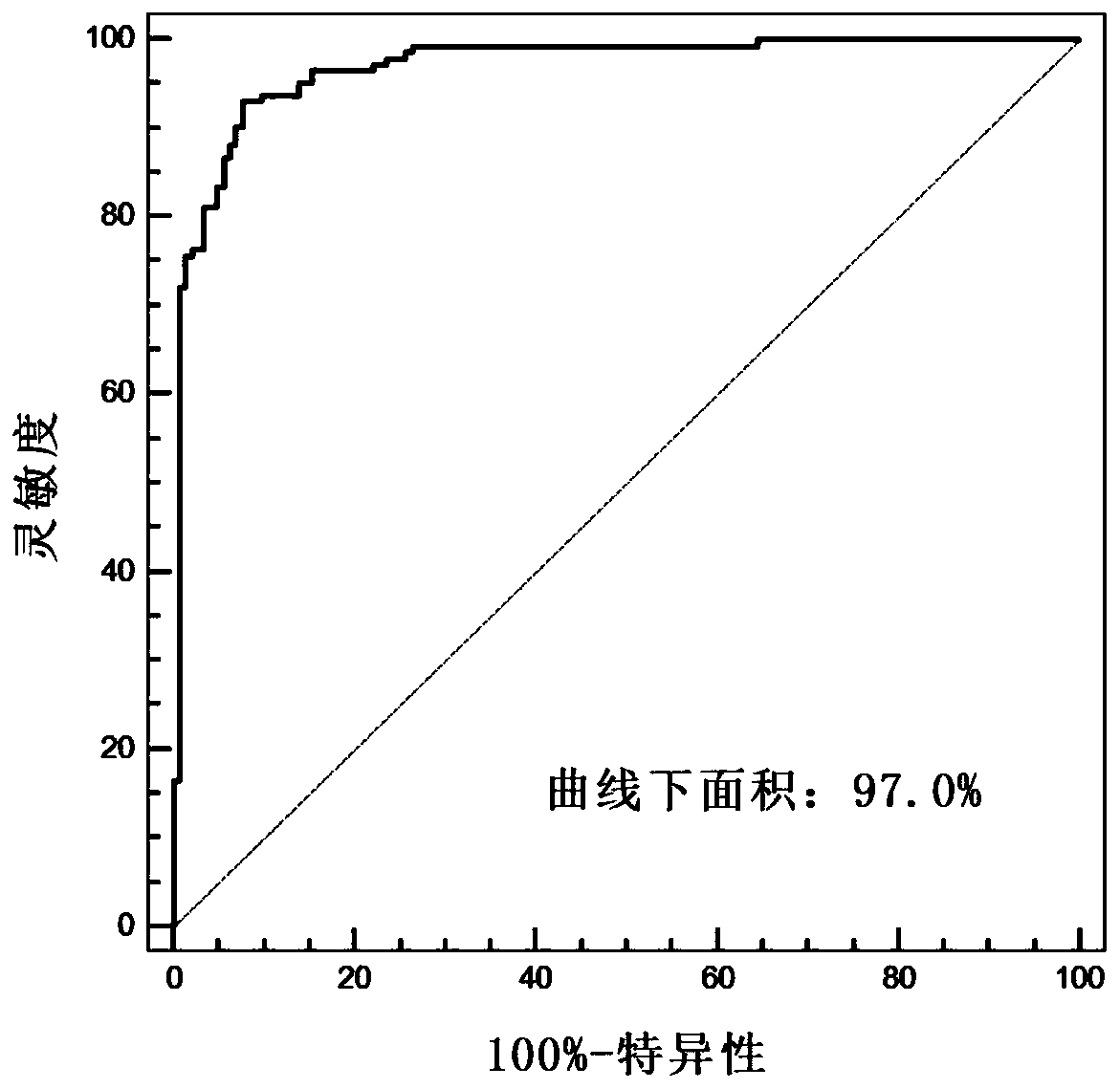
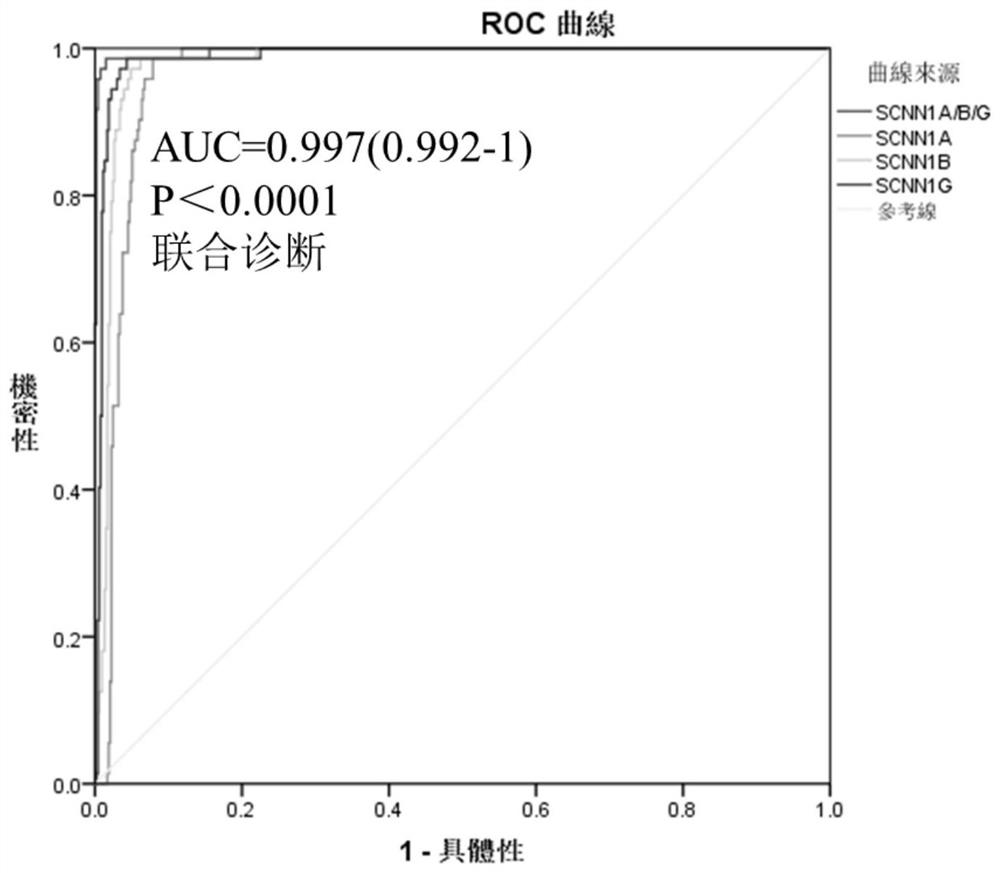
The invention discloses application of a novel molecular marker to the preparation of a kit for diagnosis and prognosis of renal clear cell carcinoma. The RNA sequence of the novel molecular marker isshown in SEQ ID NO: 1 to SEQ ID NO: 57. A mathematical model for diagnosis of renal clear cell carcinoma is constructed by taking the marker as a base; the model is high in sensitivity and good in specificity, AUC can be as high as 0.997, and the diagnosis effect is good. In addition, 57 tRF fragments can serve molecular markers for classifying renal clear cell carcinoma and predicating the survival periods of patients; in test data, a renal clear cell carcinoma tumor sample is clustered into 3 subtypes according to rRFs expression, and survivorship curve analysis shows that the survival periods of the subtypes have obvious difference. The novel tRF molecular marker has excellent diagnosis index characteristic, can be effectively applied to the diagnosis, classification and prognosis of renal clear cell carcinoma, and has high clinical application and promotion values.
Owner:ZHEJIANG UNIV
Diagnostic marker-C16orf74 gene of renal clear cell carcinoma
ActiveCN108531607AProvide survival rateMicrobiological testing/measurementBiological material analysisRenal clear cell carcinomaBiomarker (petroleum)
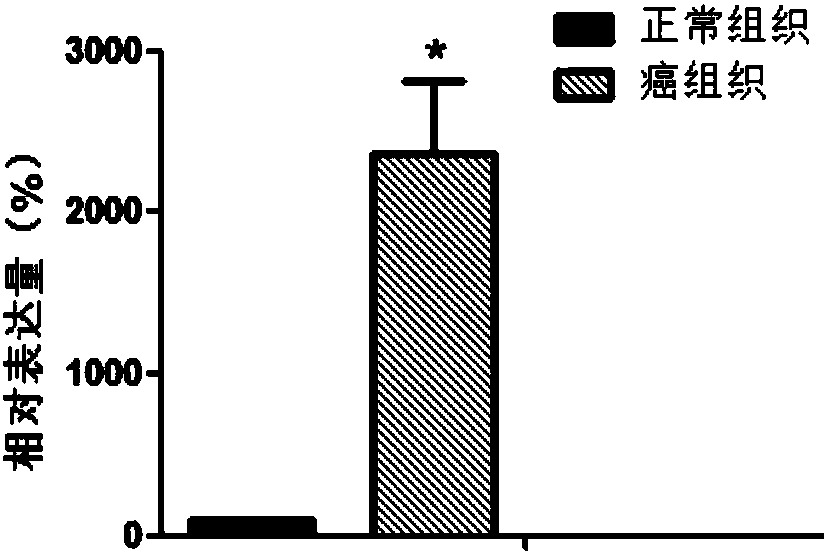
The invention discloses a C16orf74 gene which can be taken as a biomarker for the renal clear cell carcinoma. The experiment proves that compared with normal nephridial tissues, the C16orf74 gene expression in renal clear cell carcinoma tissues is significantly improved. According to the research result, the C16orf74 gene can be applied to research and development of a kit used for diagnosing therenal clear cell carcinoma and also can be used for researching and developing medicines capable of inhibiting the C16orf74 gene expression, thereby achieving clinical prevention and treatment for therenal clear cell carcinoma.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Cyclic snoRNA biomarker used for diagnosing renal clear cell carcinoma, kit and applications
ActiveCN110229912AAvoid damageReduce medical costsMicrobiological testing/measurementDNA/RNA fragmentationDiseaseRenal clear cell carcinoma
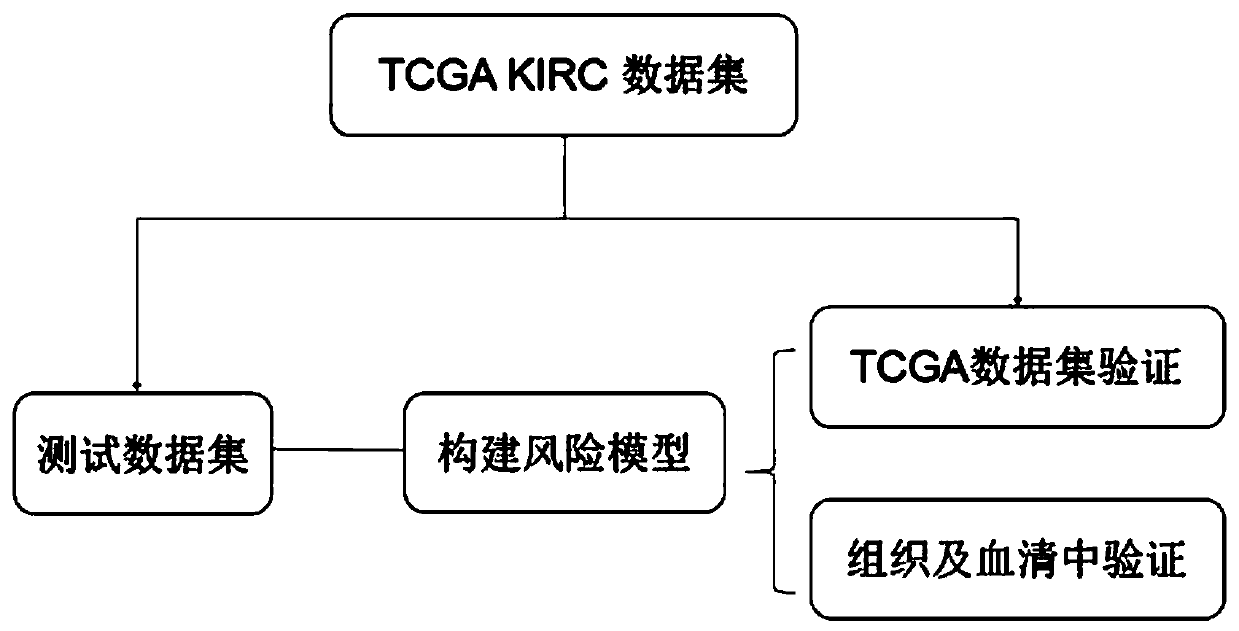
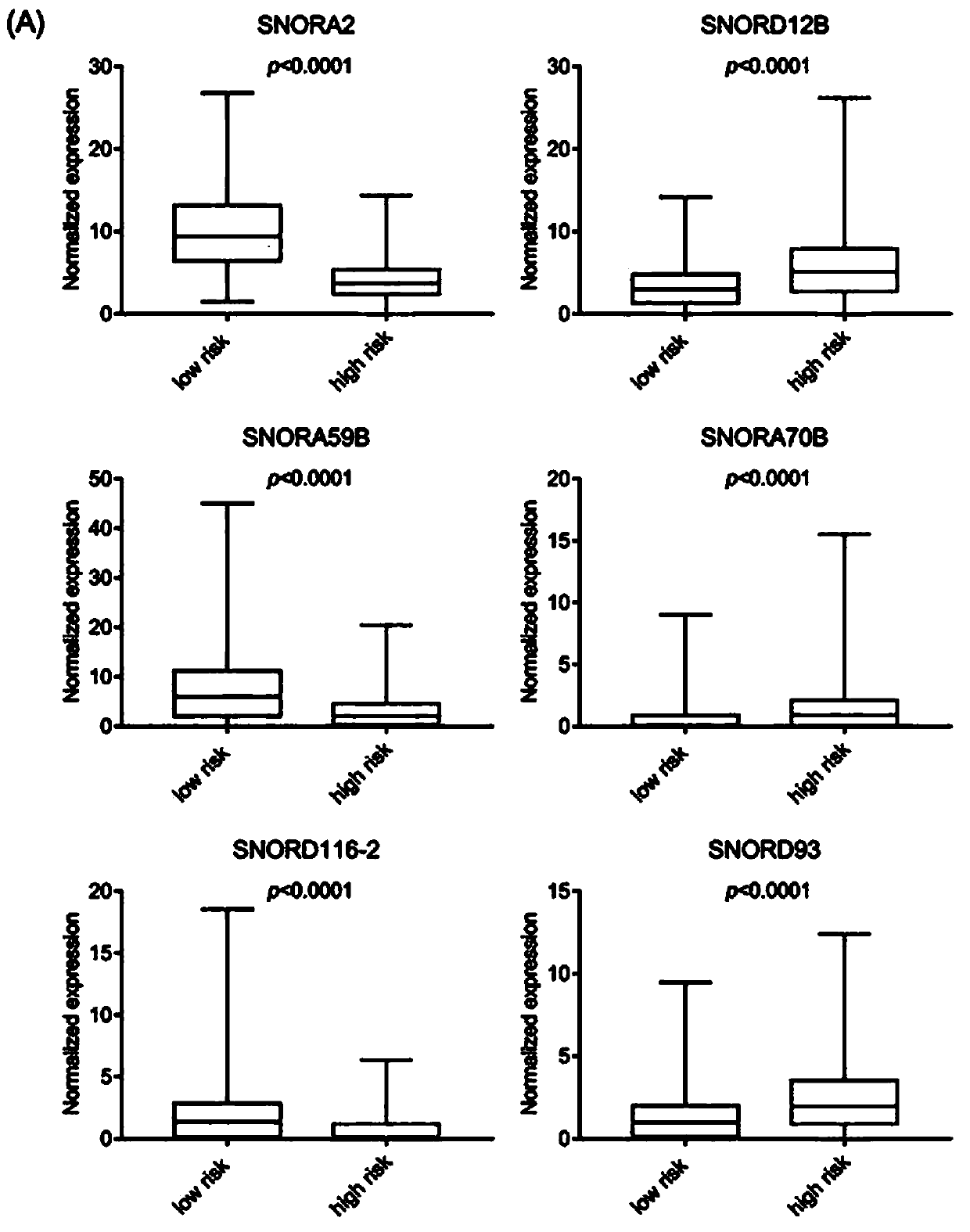
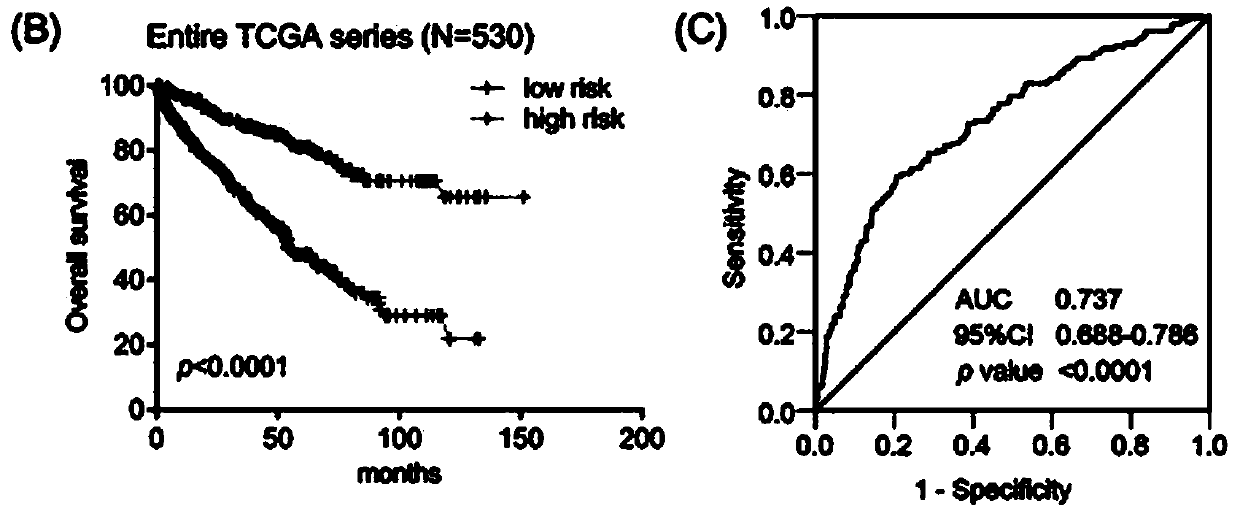
The invention belongs to the field of biological detection, and specifically relates to a cyclic snoRNA biomarker used for diagnosing renal clear cell carcinoma, a kit and applications. The biomarkerconsists of the following cyclic snoRNA: SNORA2, SNORD12B, SNORA59B, SNORA70B, SNORD93 and SNORD116-2. The snoRNA that can obviously influence the lifetime of renal clear cell carcinoma patients can be screened, and differential expression and diagnosis capabilities can be verified in tissues and serum; and the biomarker can be effectively used for the diagnosis and prognosis of renal clear cell carcinoma, and the development and utilization of the biomarker can provide a novel direction for the diagnosis of tumor and other diseases and further treatment.
Owner:中国医科大学
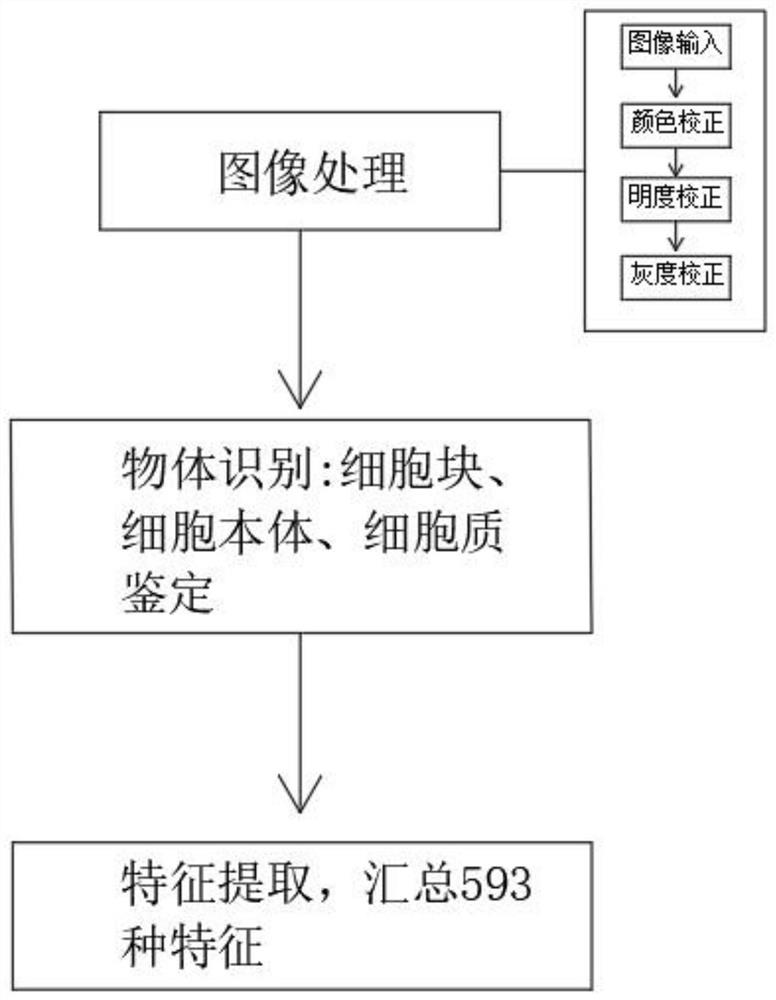
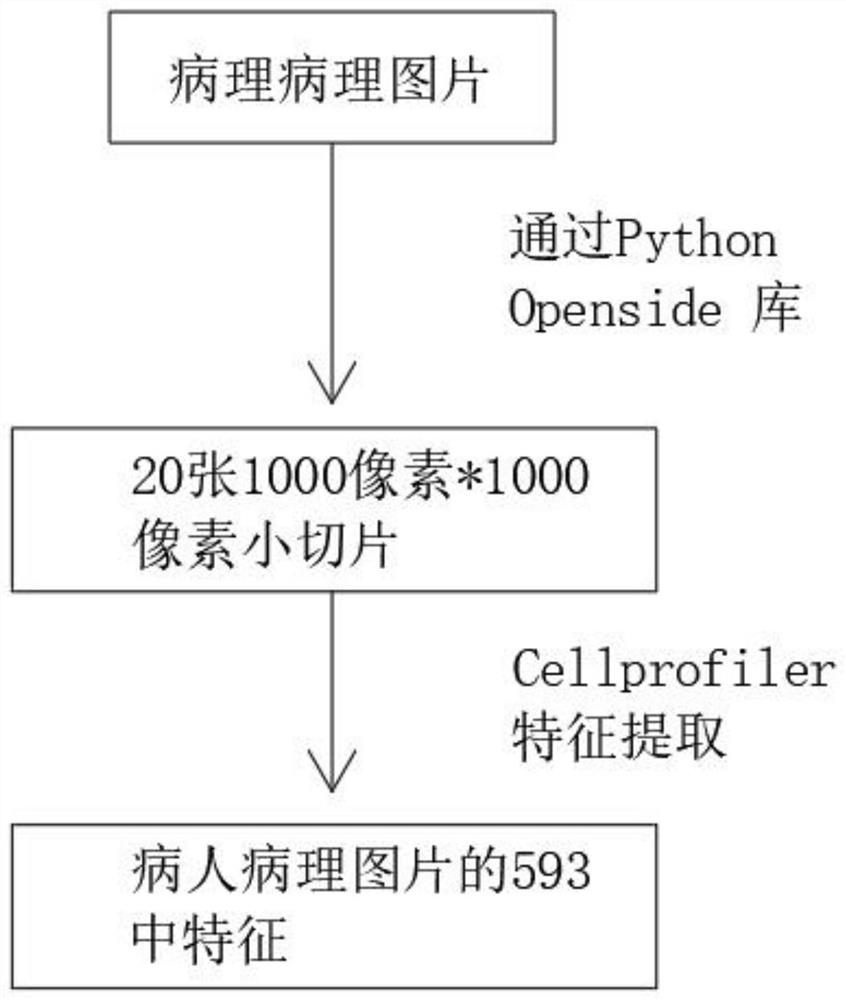
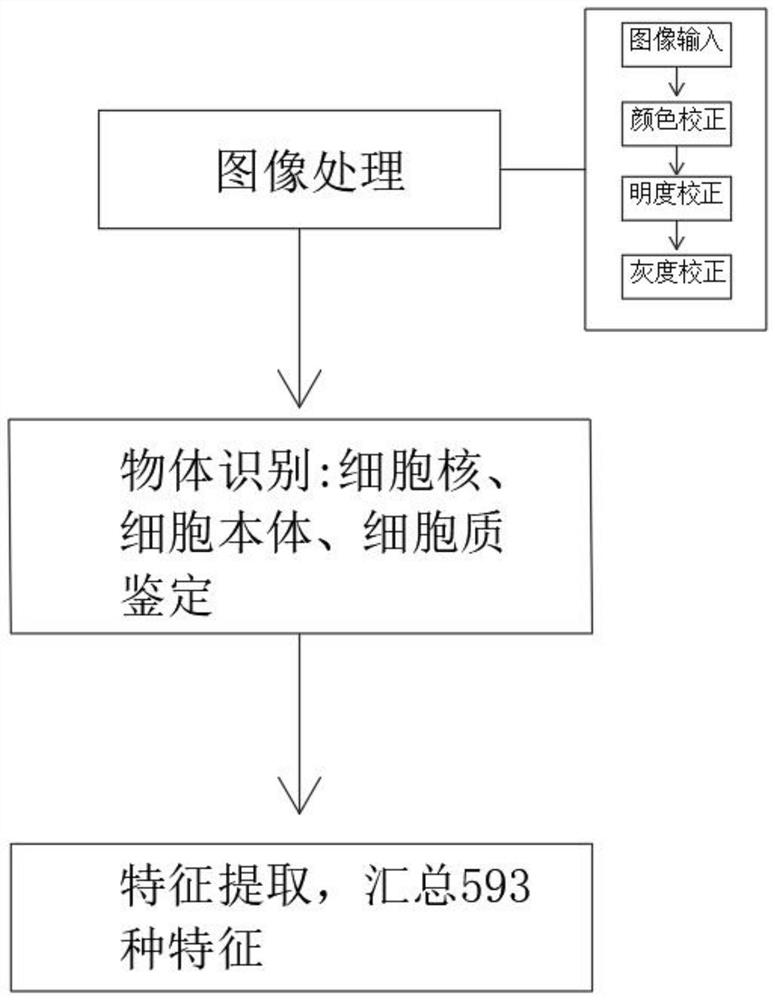
Artificial intelligence pathological diagnosis method for renal clear cell carcinoma based on deep learning
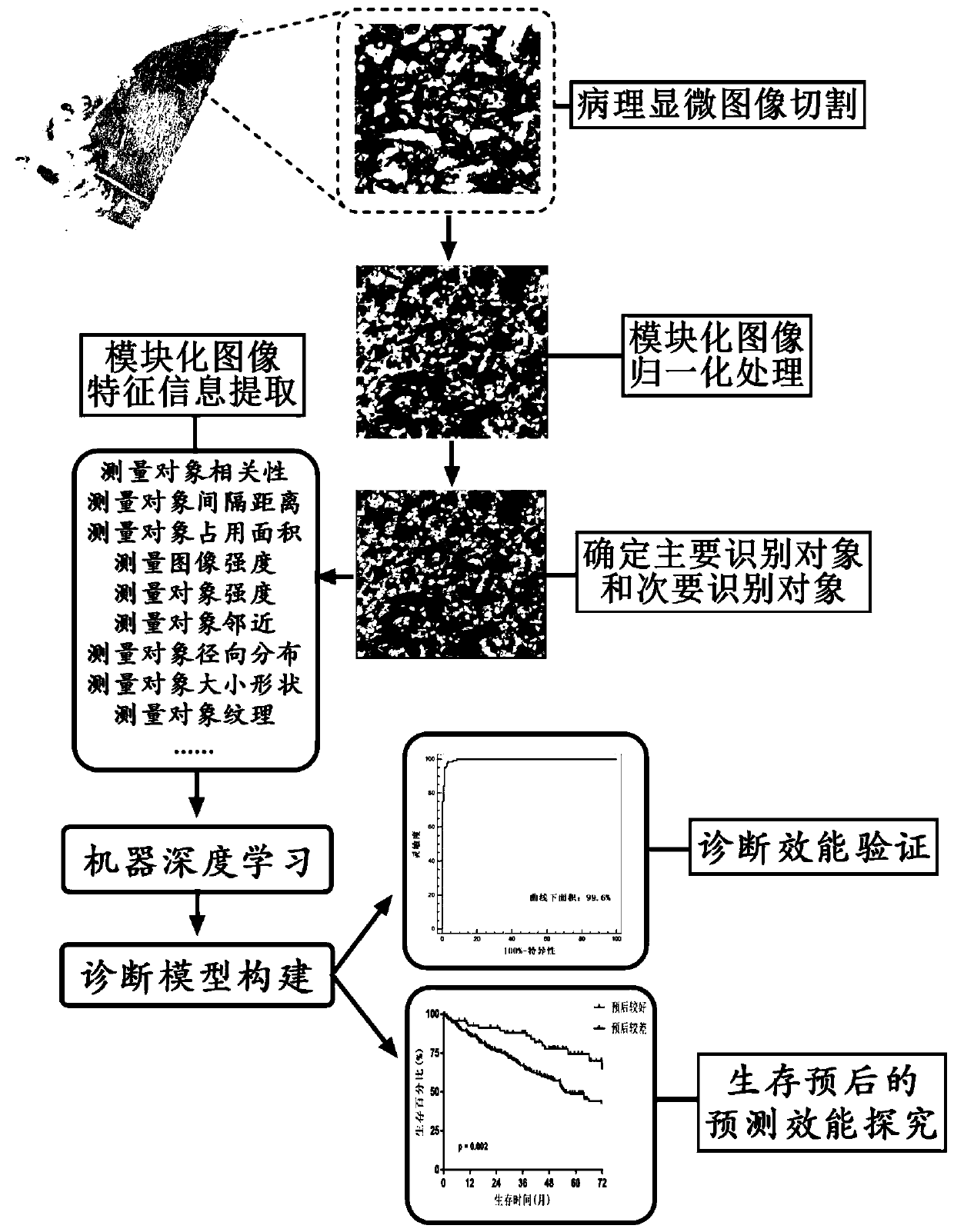
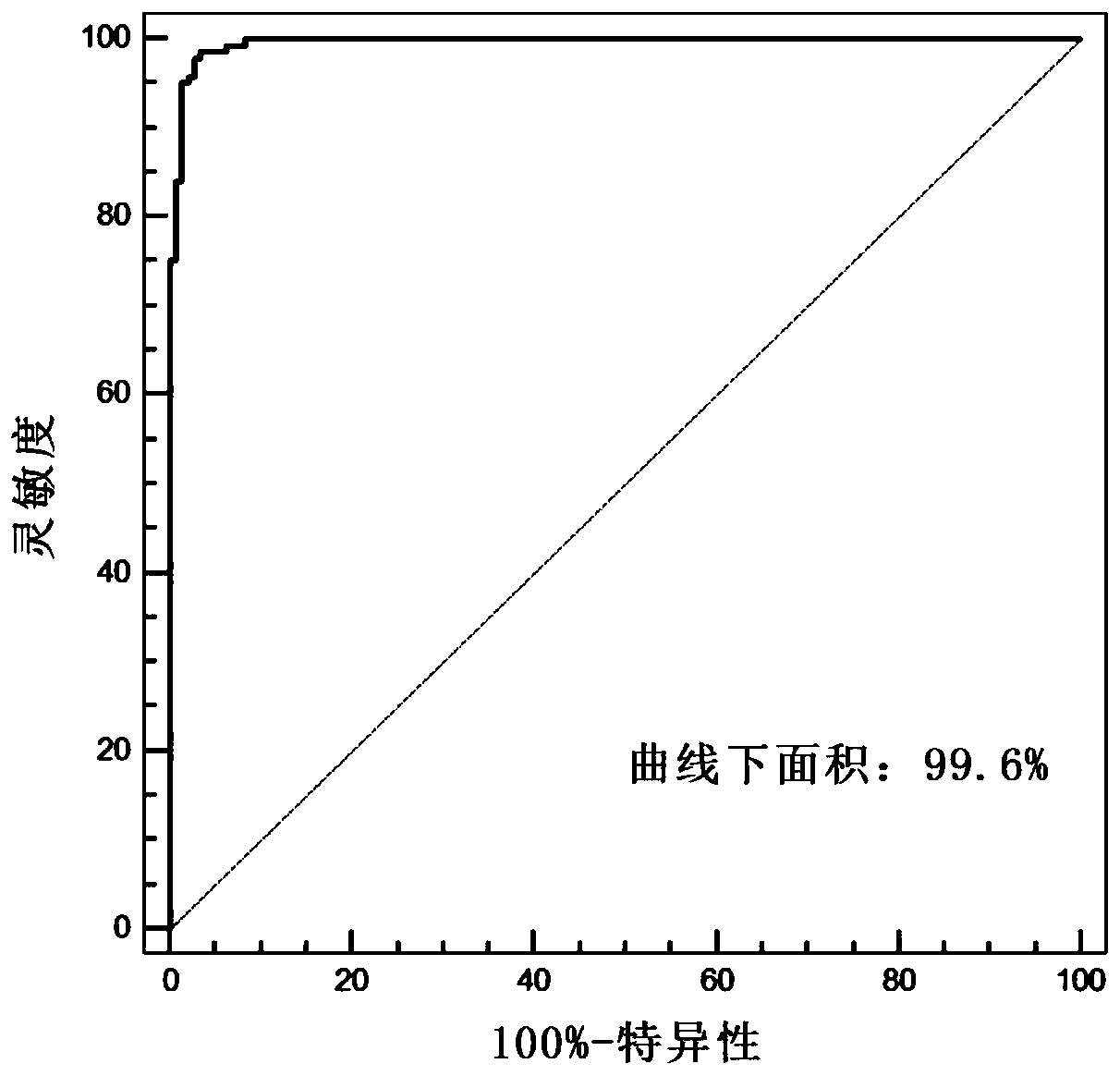
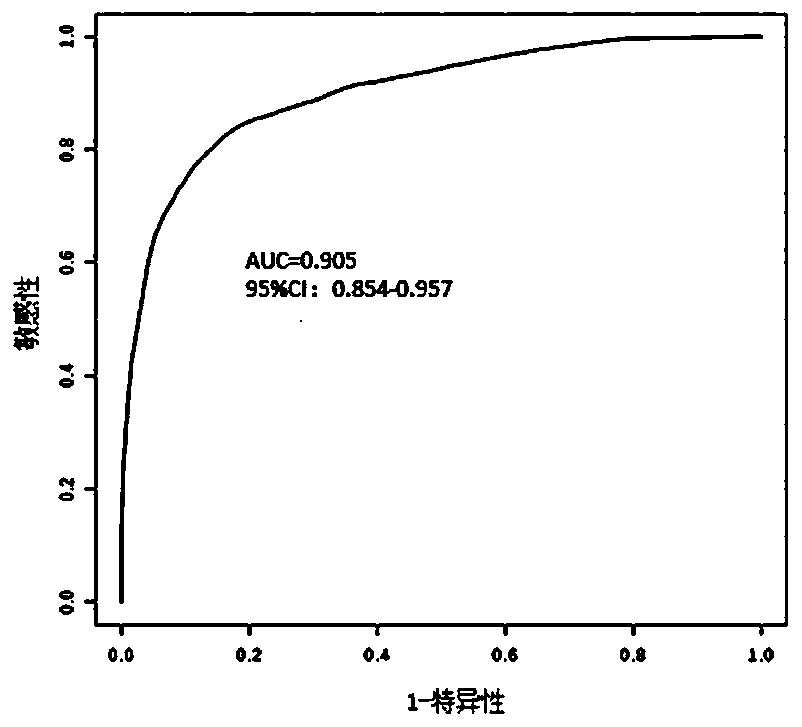
PendingCN111554381ASolve the problem of uneven pathological diagnosisEffectively predict survival prognosisImage enhancementImage analysisMicroscopic imageRenal clear cell carcinoma
The invention relates to an artificial intelligence pathological diagnosis method for renal clear cell carcinoma based on deep learning. The method comprises the following steps: S1, data acquisition;S2, pathological microscopic image processing; S3, modular image feature information extraction; S4, machine deep learning and diagnosis model construction; S5, diagnosis efficiency verification of the artificial intelligence diagnosis model: taking the artificial intelligence diagnosis model constructed by the image data in the training set as a diagnosis classifier, inputting the feature information data extracted by the test set for prediction, and evaluating the diagnosis efficiency of the artificial intelligence diagnosis model through a subject working feature curve; and S6, predictionefficiency research of survival prognosis of patients with renal clear cell carcinoma. The invention further provides a renal clear cell carcinoma artificial intelligence pathological diagnosis modelbased on deep learning. The method can effectively predict the survival prognosis of patients with renal clear cell carcinoma, can achieve the effect that cannot be achieved by traditional film reading diagnosis of pathologists, and can provide effective guidance opinions for judging whether the patients with renal clear cell carcinoma continue to be treated or not after operations.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Genome recombination fingerprint for characterizing hHRD homologous recombination deficiency and identification method thereof
The invention relates to a novel method for identifying homologous recombination deficiency and application thereof. More particularly, the present invention relates to a characteristic genome recombination fingerprint which is related to hHRD type homologous recombination repair deficiency and is resolved by using high throughput genome re-sequencing, bioinformatics analysis and statistical correlation analysis. The characteristic hHRD recombination fingerprint is caused by the functional deficiency of a specific homologous recombination repair mechanism (namely CRL4WDR70-H2B mono-ubiquitination pathway), and comprises the total frequency of genome recombination in a single sample, the composition of chromosome structure variation types and site-specific copy number variation. The recombinant fingerprint is used for identifying infectious diseases or tumors with the characteristic mutant fingerprint, and is used for guiding targeted drug treatment of PARP inhibitors. The invention relates to the method and application thereof for diseases including but not limited to breast cancer, ovarian cancer, endometrial (like) cancer, ovarian clear cell cancer, prostate cancer, pancreatic cancer, skin cancer and gastric cancer with such characteristics, as well as hepatitis B virus infection or related liver fibrosis cirrhosis, liver cancer and cholangiocarcinoma.
Owner:成都吉诺迈尔生物科技有限公司 +1
Multi-gene expression characteristic spectrum-based individualized prognosis assessment method for clear cell carcinoma of kidney
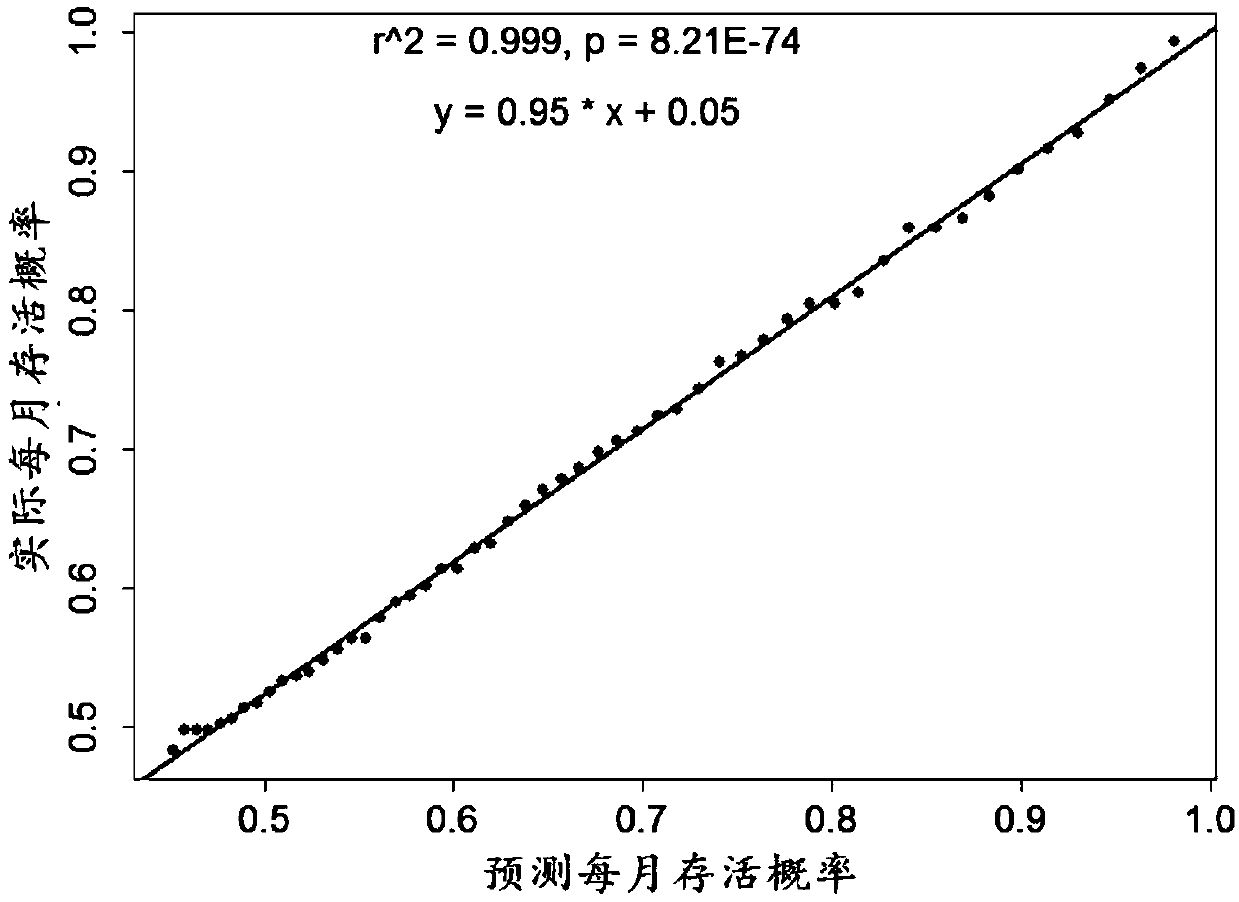
The invention discloses a multi-gene expression characteristic spectrum-based individualized prognosis assessment method for clear cell carcinoma of kidney. The method comprises the following steps of: obtaining a clear cell carcinoma of kidney prognosis risk gene list and a gene weight; constructing a prognosis assessment model by utilizing tumor tissue transcriptomes and survival data of a patient with clear cell carcinoma of kidney; calculating a risk score of the patient according to gene expression profile of tumor tissues of the patient with the clear cell carcinoma of kidney; and calculating an annual survival probability of the patient according to the risk score of the patient. According to the method, the annual survival probabilities of patients with the clear cell carcinoma ofkidney are highly consistent with the practical annual survival rates (linear dependence R2=0.999, P=8.21E-74), so that the method has high prediction correctness and is highly identical with practical survival states; and meanwhile, for each tumor patient, the method is capable of giving s peculiar survival probability curve of the patient.
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Application of metabolic marker in renal clear cell carcinoma
InactiveCN110286223AAchieve early diagnosisImprove the quality of lifeComponent separationMass spectrometric analysisBacteriuriaMetabolite
The invention discloses an application of a metabolic marker in renal clear cell carcinoma. According to the invention, metabonomics analysis is carried out by collecting renal clear cell carcinoma and urine samples of normal people, and metabolites with significant differences in different groups are found, so that early renal clear cell carcinoma can be predicted by using the metabolites, and then personalized treatment of patients is realized.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
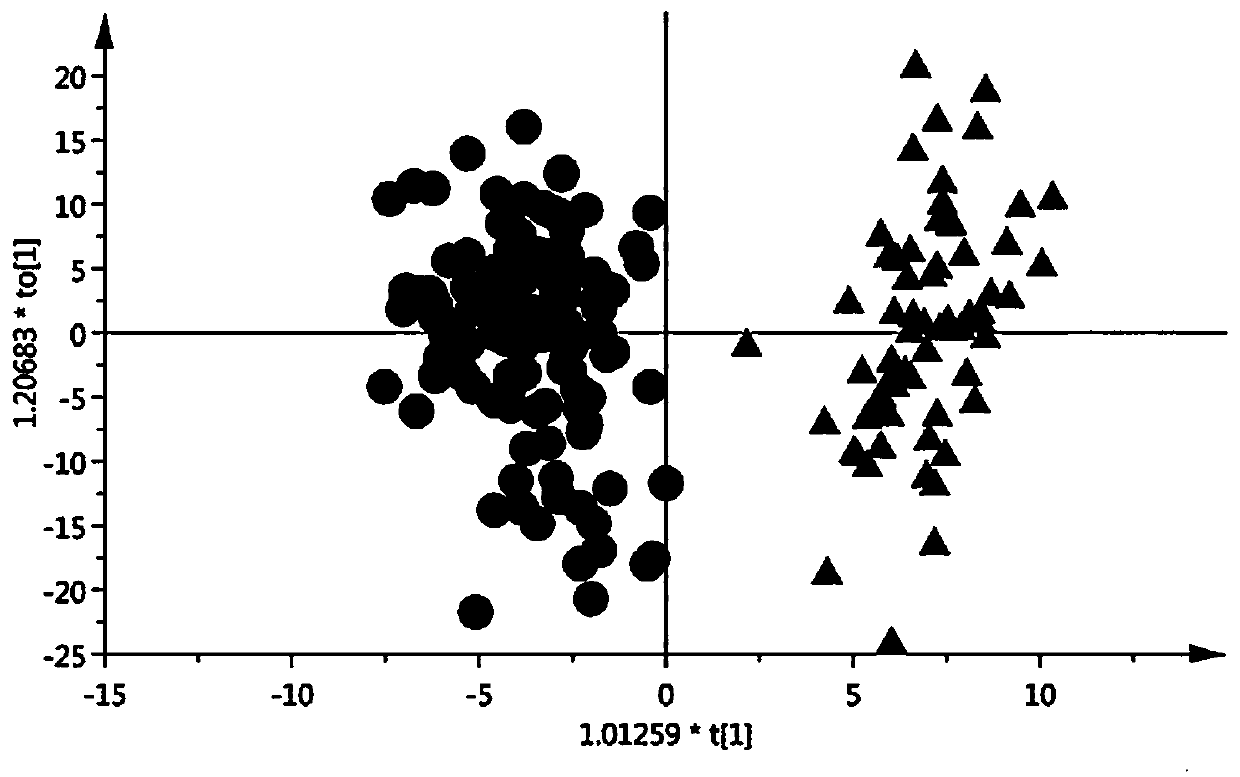
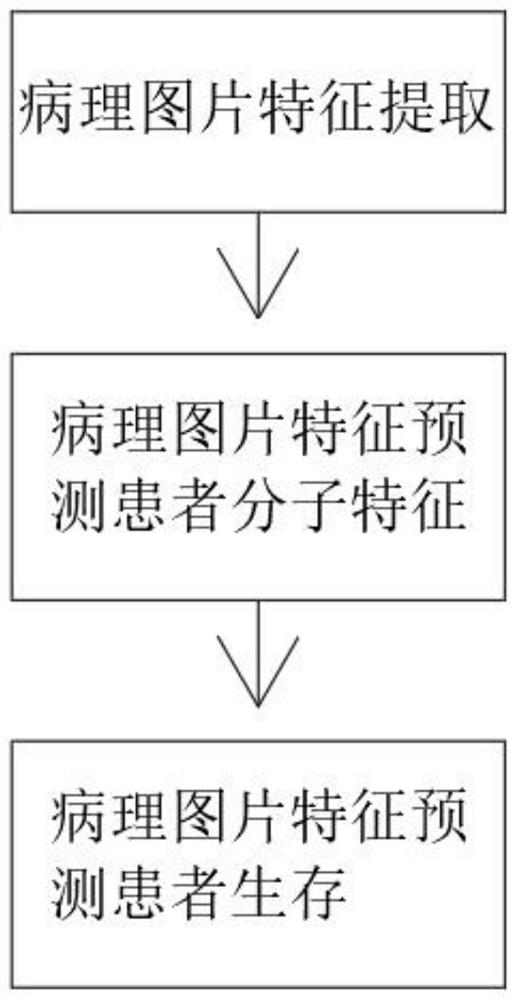
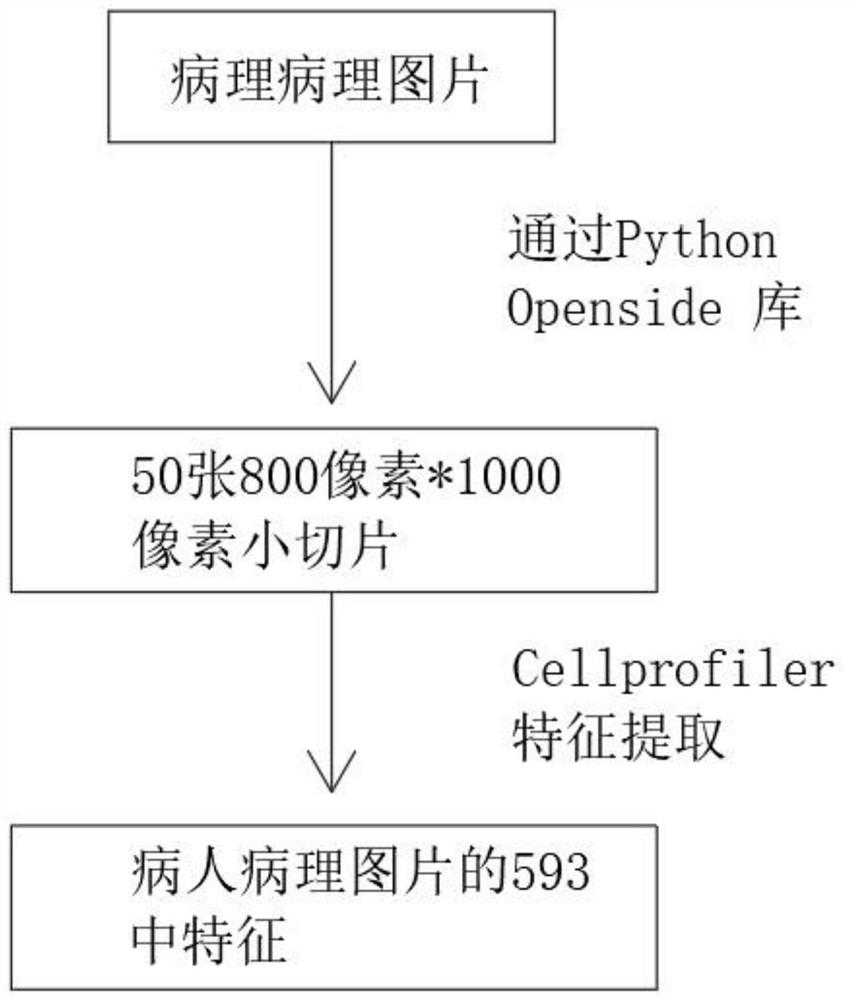
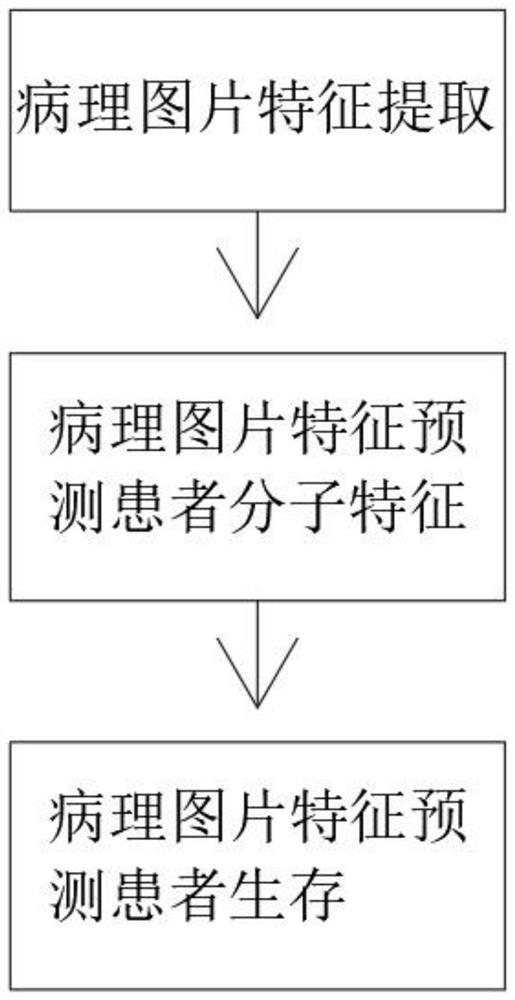
Pathological picture-based renal clear cell carcinoma molecular feature prediction and prognosis technology
PendingCN113436722ARapid economic quantificationHealth-index calculationEpidemiological alert systemsPatient survivalRenal clear cell carcinoma
The invention discloses a pathological picture-based renal clear cell carcinoma molecular feature prediction and prognosis technology. The renal clear cell carcinoma molecular feature prediction and prognosis technology comprises the following parts: 1, feature extraction of the pathological picture; 2, predicting the molecular characteristics of the patient through the pathological picture; 3, predicting the lifetime of the patient by integrating a single pathological picture and a pathological picture with multiple omics; according to the method, the pathological picture of the patient can be quickly and economically quantified, and the survival time of the patient with existing gene, transcription or proteomics data can be quickly, economically and more accurately judged when the important mutation state, molecular subtype attribution and survival of the patient are predicted.
Owner:曾皓
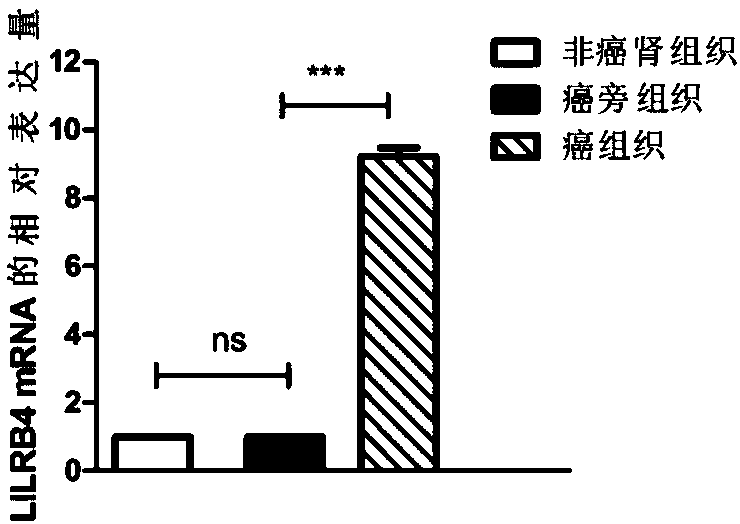
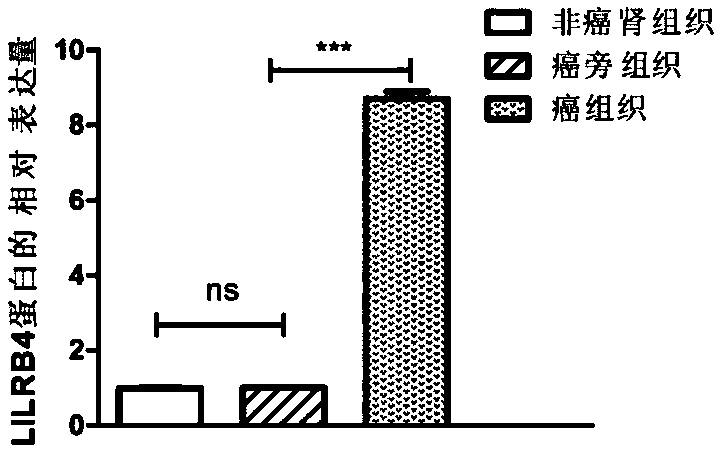
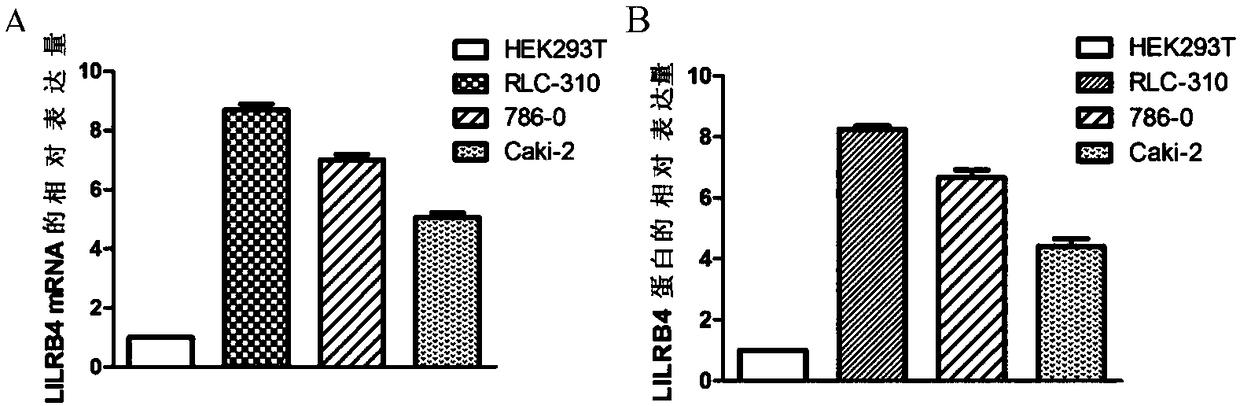
Biological marker related with clear cell renal cell carcinoma and application thereof
ActiveCN108085393ACompound screeningApoptosis detectionClear cell renal cell carcinomaBiomarker (petroleum)
The invention discloses a biological marker related with clear cell renal cell carcinoma and application thereof. The biological marker is LILRB4. The invention discloses a reagent, a kit and a chip for detecting the level of LILRB4, a medicine composition with down-regulated level of LILRB4, and application of the reagent, the kit and the chip or the medicine composition in preparation of products for diagnosing or treating the clear cell renal cell carcinoma and the invasion and transfer of the clear cell renal cell carcinoma.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
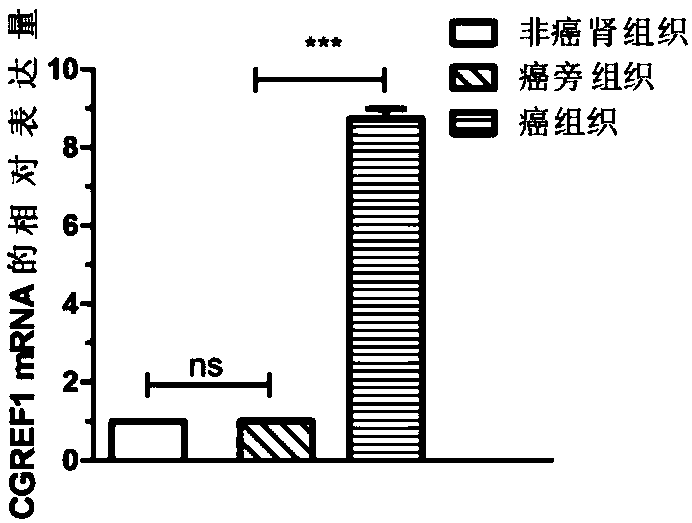
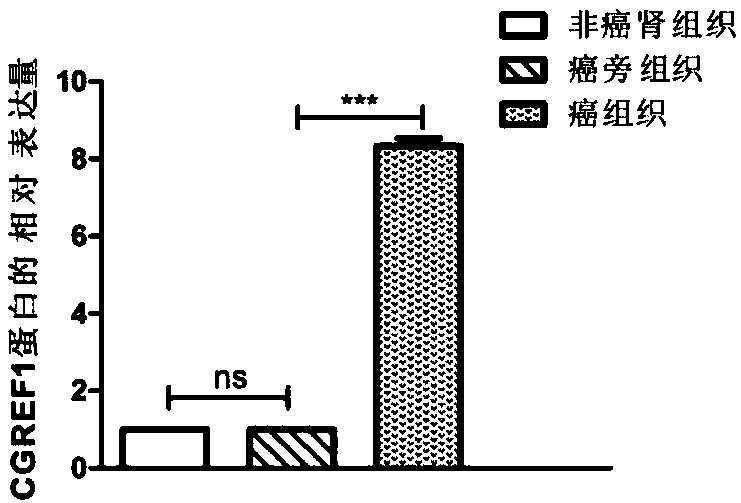
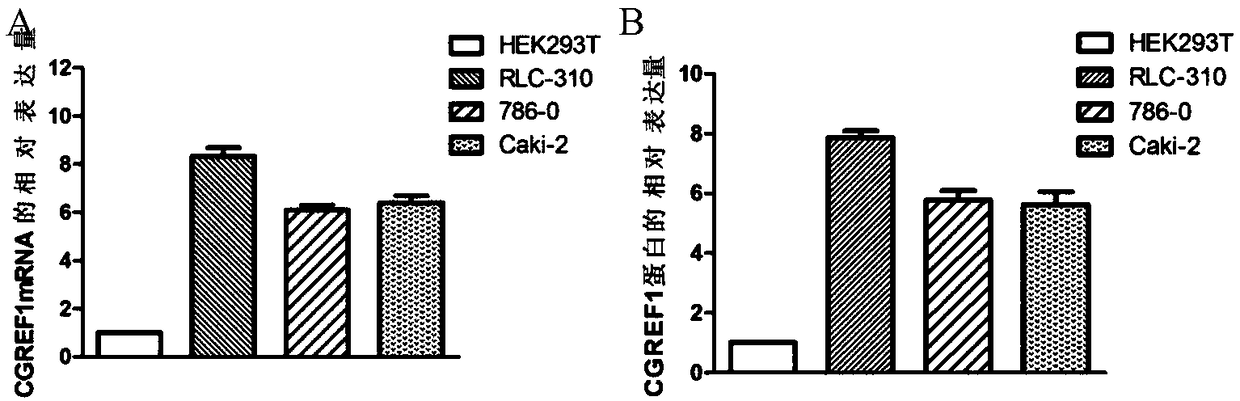
Application of CGREF1 as marker in diagnosis and treatment of renal clear cell carcinoma
PendingCN108220443AImprove accuracyPrevent invasionMicrobiological testing/measurementMaterial analysisRenal clear cell carcinomaOncology
The invention discloses application of CGREF1 as a marker in diagnosis and treatment of renal clear cell carcinoma. The invention discloses a product including a reagent for detecting the expression level of the CGREF1 and application of relevant reagents in preparing the product for diagnosing the renal clear cell carcinoma; meanwhile, the invention discloses a pharmaceutical composition including an inhibitor for inhibiting the expression level of the CGREF1 and application of relevant inhibitors in preparing the pharmaceutical composition for treating the renal clear cell carcinoma; the invention also discloses application of the CGREF1 in screening candidate compounds for treating the renal clear cell carcinoma.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Kidney clear cell carcinoma molecular feature prediction and prognosis technology based on pathological pictures
InactiveCN112489813AHigh-precision output prognostic risk scoreRapid economic quantificationProteomicsGenomicsPatient survivalRenal clear cell carcinoma
The invention discloses a renal clear cell carcinoma molecular feature prediction and prognosis technology based on a pathological picture. The renal clear cell carcinoma molecular feature predictionand prognosis technology comprises the following steps: 1, carrying out feature extraction of the pathological picture; 2, predicting the molecular characteristics of the patient through the pathological picture; 3, integrating a single pathological picture and a pathological picture into multiple omics to predict the lifetime of the patient. According to the method, pathological pictures of patients can be quantified quickly and economically, important mutation states, molecular subtype affiliation and survival time of the patients can be predicted, and the survival time of the patients withexisting gene, transcription or proteomics data can be judged quickly, economically and more accurately.
Owner:曾皓
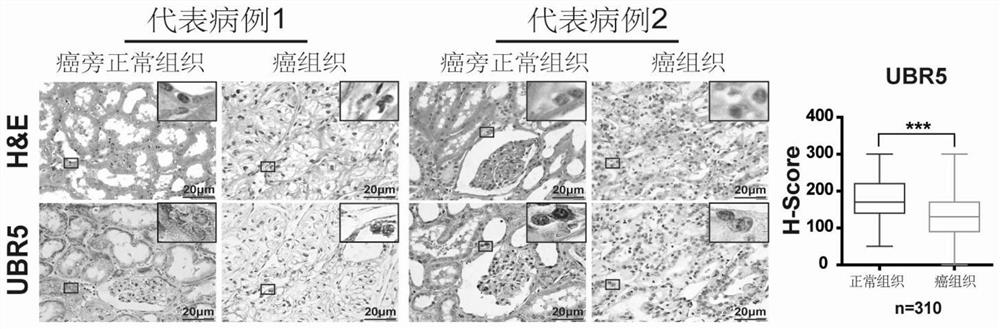
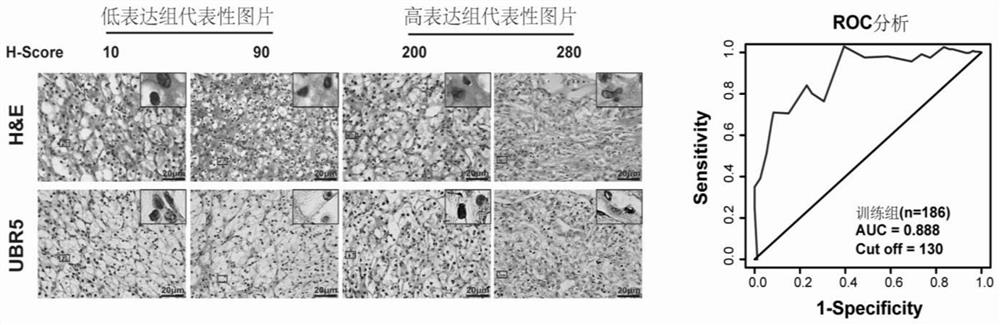
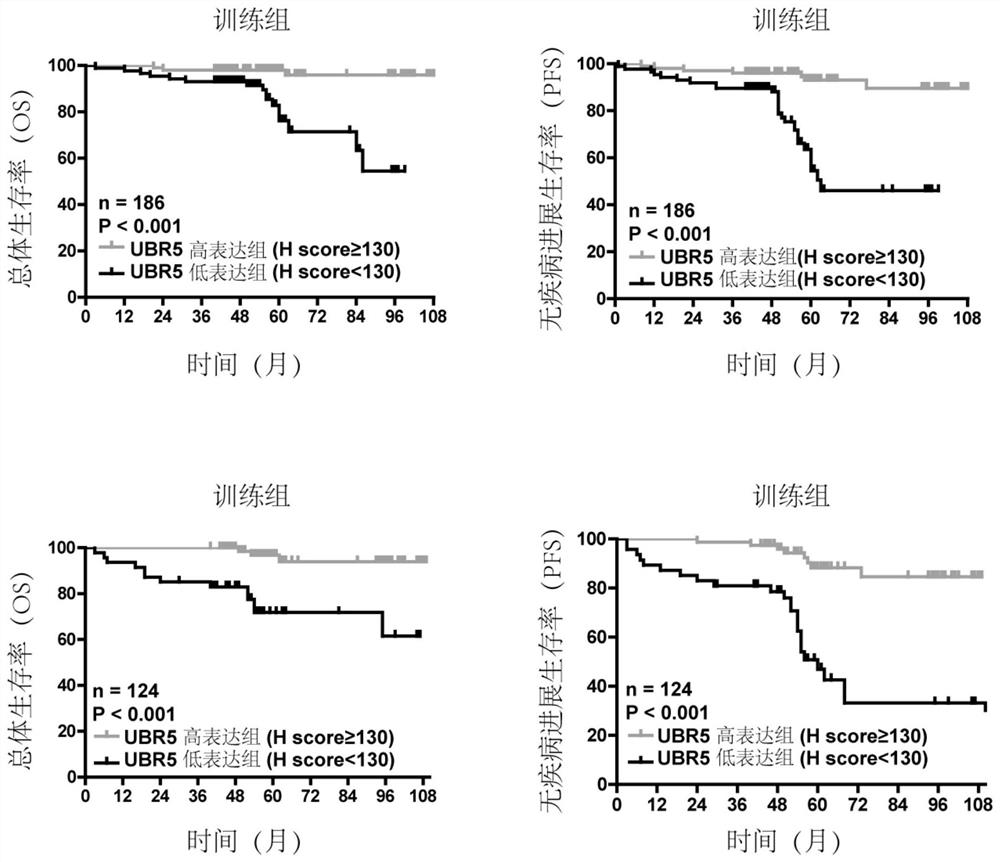
Application of E3 ubiquitination protein ligase UBR5 in preparation of tumor diagnosis or prognosis evaluation kit
PendingCN111912985APrecise riskAccurate Prognosis PredictionMaterial analysisProgression-free survivalUbiquitinated Proteins
The invention relates to the field of biotechnology and medical diagnosis, in particular to application of E3 ubiquitination protein ligase UBR5 in preparation of a renal clear cell carcinoma diagnosis or prognosis evaluation reagent or kit and a corresponding detection kit. Researches find that the expression of E3 ubiquitination protein ligase UBR5 molecules in patients with renal clear cell carcinoma is generally reduced, the expression level of the E3 ubiquitination protein ligase UBR5 molecules is remarkably related to prognosis of the patients, and the total lifetime (OS) and progression-free lifetime (PFS) of the patients with renal clear cell carcinoma can be better predicted by combining the expression of UBR5.
Owner:SHANGHAI CITY PUDONG NEW AREA GONGLI HOSPITAL
Separation and culture method of mouse kidney clear cell carcinoma circulating tumor cell line and human kidney clear cell carcinoma circulating tumor cells
ActiveCN111321120AThe method of isolation and culture is simpleEasy to operateCell culture active agentsTumor/cancer cellsRenal clear cell carcinomaOncology
The invention discloses a separation and culture method of a mouse kidney clear cell carcinoma circulating tumor cell line and kidney clear cell carcinoma circulating tumor cells, and relates to the technical field of separation and culture of tumor cells. The mouse kidney clear cell carcinoma circulating tumor cell line is circulating tumor cells of an in-situ kidney cancer NOD / SCID mouse. The separation and culture method of the circulating tumor cells mainly comprises the following steps of: firstly, infecting a human kidney clear cell carcinoma cell line 786-O with viruses, so that the human kidney clear cell carcinoma cell line is stably converted into green fluorescent protein and has Puromycin resistance, and culturing the cell line; and then establishing a mouse model, collecting peripheral blood of the mouse, and culturing and separating the peripheral blood to obtain the kidney cancer circulating tumor cells. The separation and culture method disclosed by the invention is simple and easy to operate, and the circulating tumor cells obtained by separation can be subjected to normal leaflet culture and can be widely applied to clinical research.
Owner:SECOND AFFILIATED HOSPITAL OF COLLEGE OF MEDICINEOF XIAN JIAOTONG UNIV
application of m6A modification related combined genome in predicting curative effect of immunotherapy for patients with renal clear cell carcinoma
ActiveCN113462776APredicted responseHigh predictive value of immunotherapy efficacyMicrobiological testing/measurementBioinformaticsTreatment effectRenal clear cell carcinoma
The invention relates to the technical field of medical biological detection, provides a new application of a combined genome of HNRNPA2B1 and ALKBH5, and particularly relates to an application in preparation of a reagent or a kit for predicting the curative effect of renal clear cell carcinoma immunotherapy. The invention also provides a renal clear cell carcinoma immunotherapy curative effect prediction kit and a renal clear cell carcinoma immunotherapy curative effect prediction system. The gene combination is derived from a renal clear cell carcinoma m6A modification mode differential expression mode, discovery of the m6A modification related gene combination model provides a brand-new strategy for predicting the immune treatment effect of a renal clear cell carcinoma patient, clinical doctors can be guided to implement an individualized precise treatment strategy, the survival rate of the patient is increased, and the method has important guiding significance for immunotherapy application of patients with renal clear cell carcinoma.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
DNA methylated biomarker related to prognosis of renal clear cell carcinoma
The invention belongs to the technical field of biomedicine, and relates to a marker for prognosis of renal clear cell carcinoma and application of the marker. According to the invention, comparative analysis is carried out on the whole genome methylation level, differential methylation sites and differential methylation regions among tumor tissues and adjacent normal tissues by performing testing observation on the relationship between the change of DNA methylation related markers in renal clear cell carcinoma and the prognosis condition of diseases, and by utilizing an LASSO-Cox regression model, biological markers related to prognosis of renal clear cell carcinoma are determined to be TP73 (cg00295572, cg07382920, cg20611911 and cg01915516) and CTBP2 (cg05749728) respectively. The DNA methylation related markers disclosed by the invention can be used for preparing a product for evaluating the prognosis of the renal clear cell carcinoma.
Owner:FUDAN UNIV +1
Characteristic miRNA expression profile combination and early prediction method for renal clear cell carcinoma
PendingCN111733251AFast predictionQuick forecastMicrobiological testing/measurementBiostatisticsEarly predictionNucleotide
The invention discloses a characteristic miRNA expression profile combination and an early prediction method for renal clear cell carcinoma. The miRNA comprises hsa-let-7g, hsa-mir-10a, hsa-mir-10b, hsa-mir-125a, hsa-mir-21, hsa-mir-30b, hsa-mir-30c-1, hsa-mir-30c-2 and hsa-mir-532, and the nucleotide probe sequence is shown as SEQ ID NO. 1-9. The evaluation of the early risk of renal clear cell carcinoma based on the miRNA expression profile combination characteristics has high accuracy and accuracy (the area AUC under the ROC curve = 0.992). The early risk of renal clear cell carcinoma can be calculated by a support vector machine model only by acquiring the relative expression quantity of the nine miRNAs.
Owner:ANHUI MEDICAL COLLEGE
Featured lincRNA expression profile combination and early stage prediction method for renal clear cell carcinoma
InactiveCN111808965AFast predictionQuick forecastMicrobiological testing/measurementBiostatisticsNucleotideRenal clear cell carcinoma
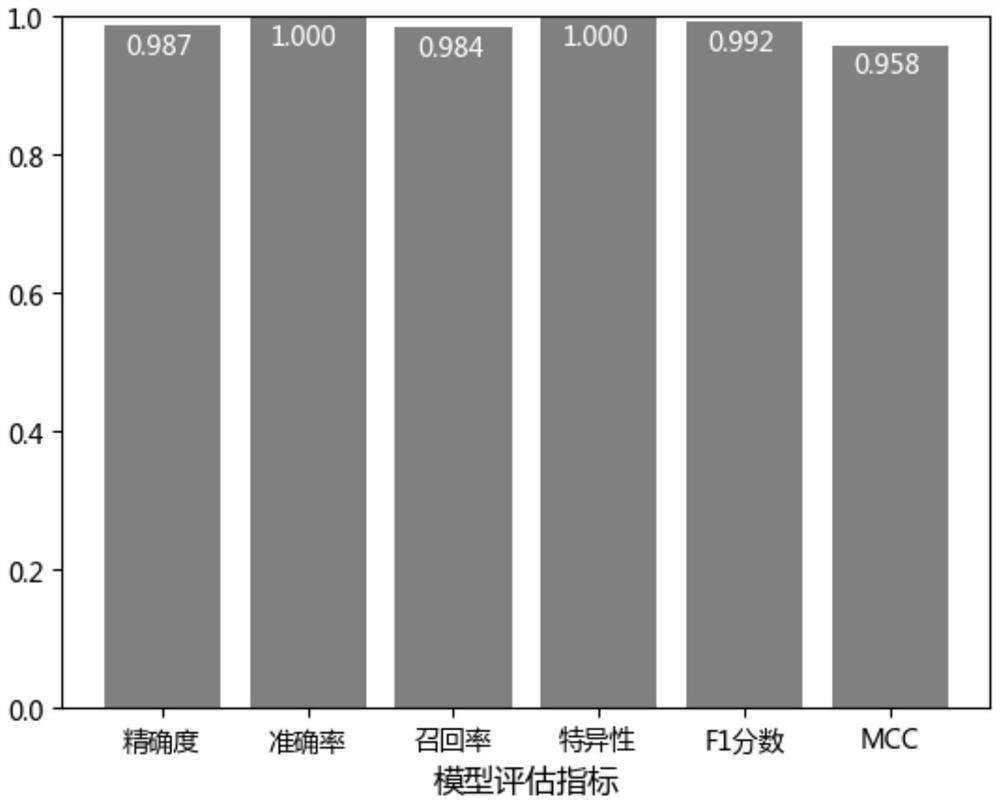
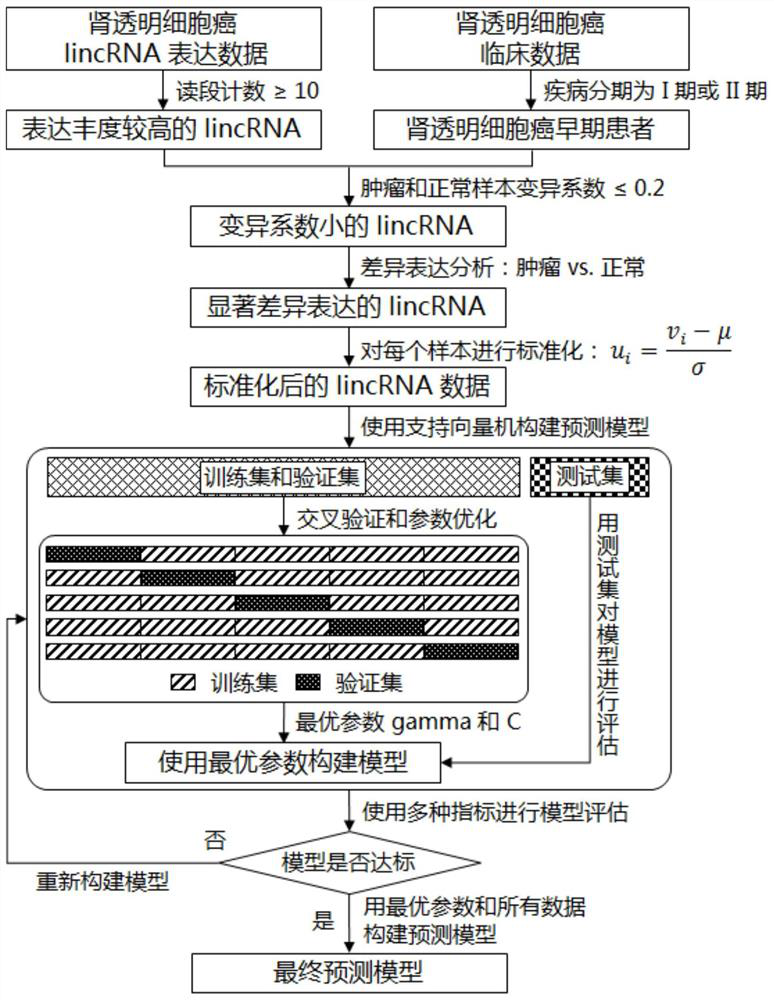
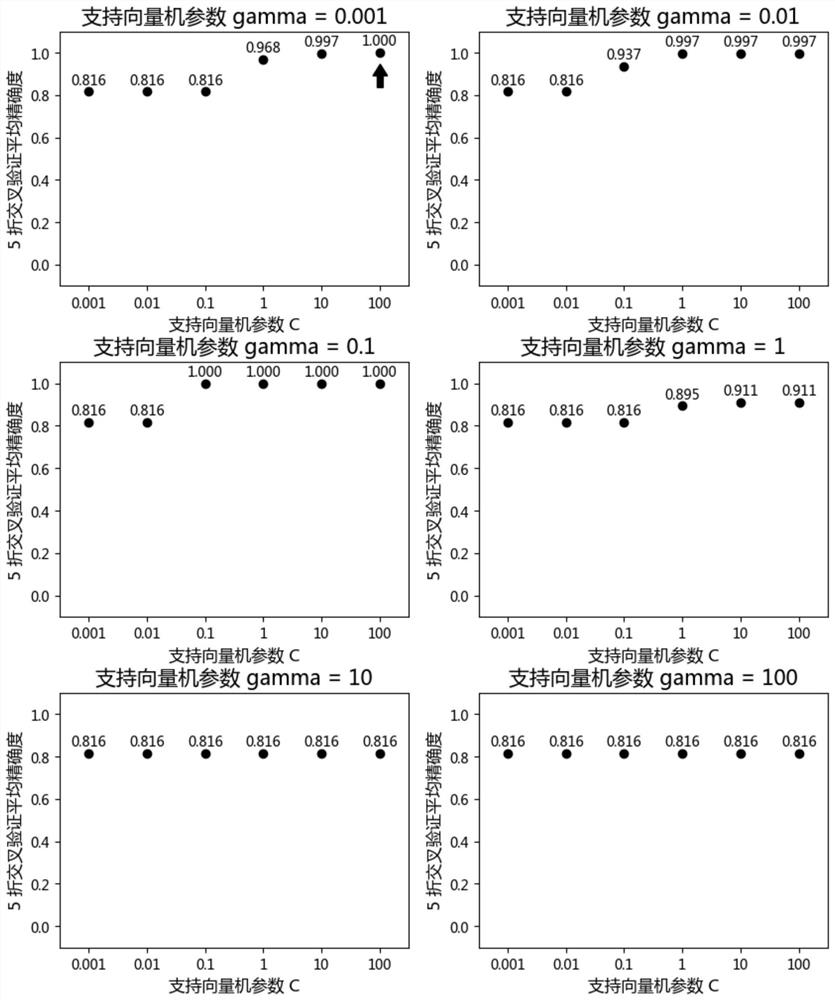
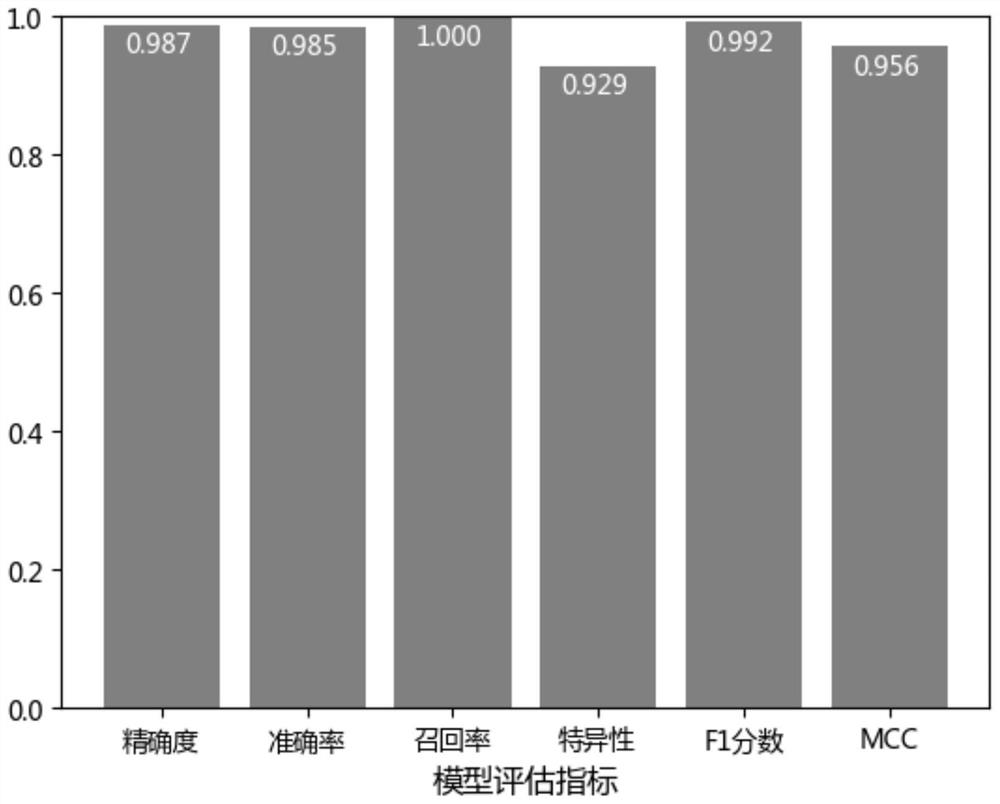
The invention discloses a featured lincRNA expression profile combination and an early stage prediction method for a renal clear cell carcinoma. The nucleotide probe sequence of the lincRNA is disclosed by SEQ ID NO.1-26. The early-stage risk assessment of the renal clear cell carcinoma on the basis of the characteristics of the lincRNA expression profile combination has high precision and accuracy (ROC (Receiver Operating Curve) ACU (Area Under The Curve) is equal to 0.964). Only the relative expression quantities of the above 26 types of lincRNA need to be obtained, an early stage illness probability of the renal clear cell carcinoma is given through a support vector machine model, and the early stage illness probability can be taken as a reference basis for the early stage prediction ofthe renal clear cell carcinoma.
Owner:FOSHAN UNIVERSITY
SCNN1 primer and diagnostic kit for clear cell nuclear cell carcinoma and application of SCNN1 primer
ActiveCN112553341AStrong specificityFast and good diagnosticsMicrobiological testing/measurementDNA/RNA fragmentationForward primerNucleotide
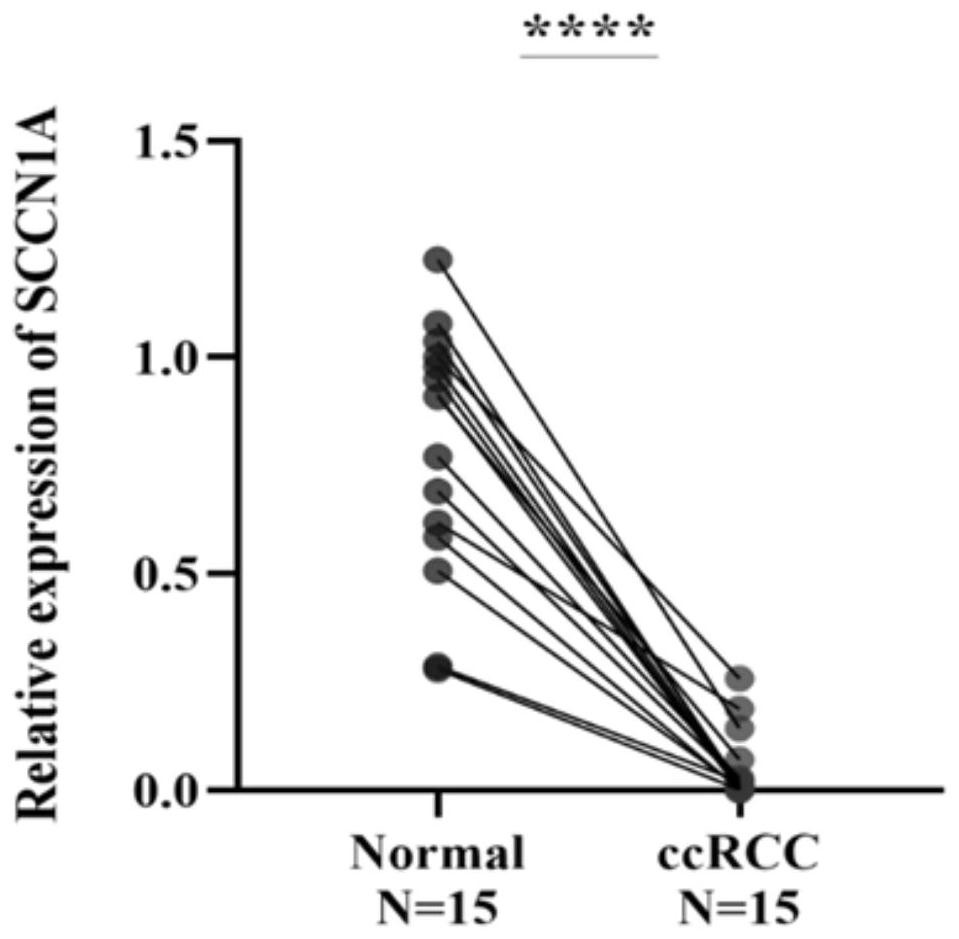
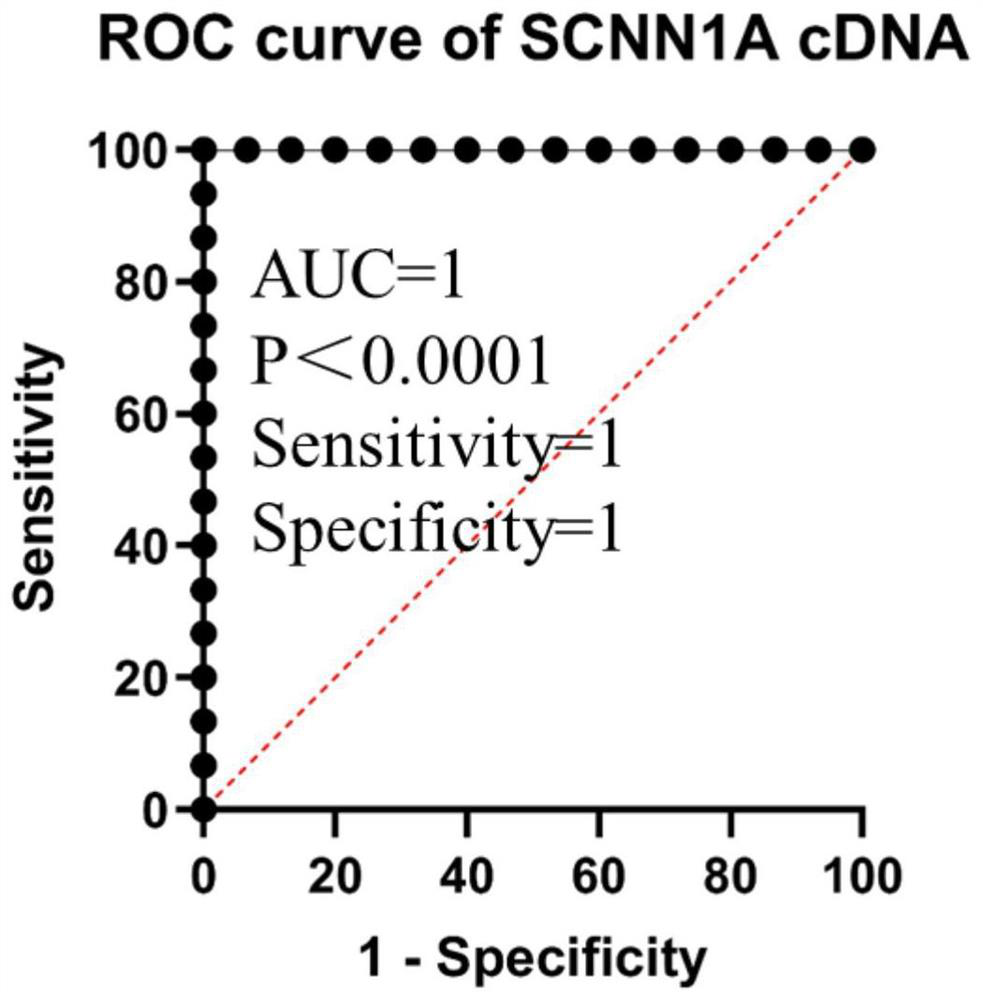
The SCNN1 primer for rapidly diagnosing clear cell nuclear cell carcinoma provided by the invention comprises an internal reference primer pair which is GAPDH, mRNA sequence forward primers and reverse primers of SCNN1A genes with nucleotide sequences shown in SEQ ID NO: 1 and SEQ ID NO: 2, mRNA sequence forward primers and reverse primers of SCNN1B genes with nucleotide sequences shown in SEQ IDNO: 3 and SEQ ID NO: 4, and mRNA sequence forward primers and reverse primers of SCNN1G genes with nucleotide sequences shown in SEQ ID NO: 5 and SEQ ID NO: 6, which take human GAPDH as an internal reference control, and forward primers and reverse primers, having nucleotide sequences shown as SEQ ID NO: 7 and SEQ ID NO: 8. A corresponding kit is also prepared. The kit comprises the following reagents: a tissue lysis solution Trizol, an SYBRGreen Master Mix reaction solution, a reverse transcription reaction solution, a negative control and a positive control. The primer and the kit can eliminate false negative and false positive of clinical sample detection, and are safe, reliable, high in accuracy and suitable for clinical popularization.
Owner:GUANGXI MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com