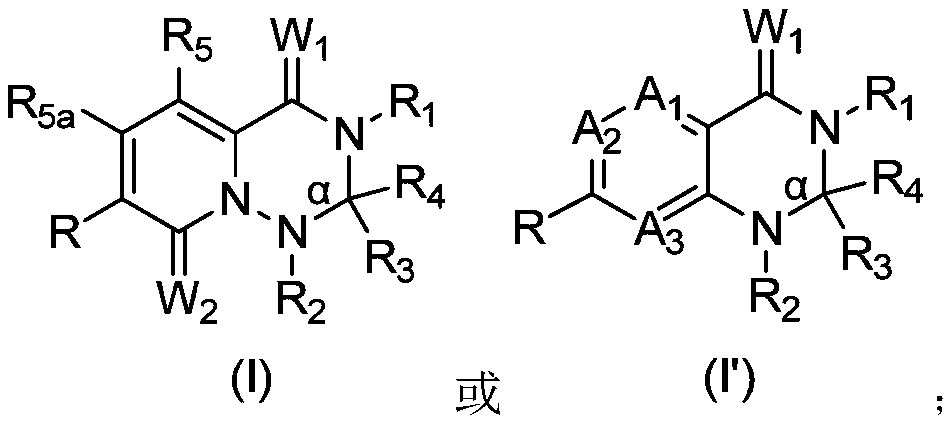
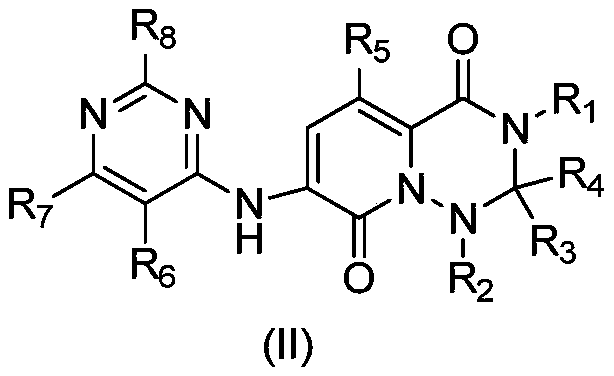
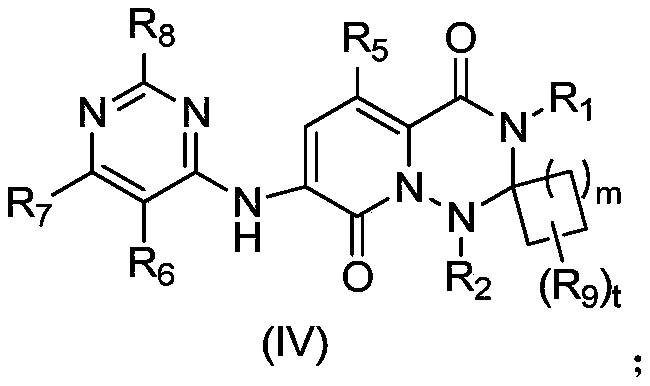
MNK inhibitors
One-NH2, compound technology, applied in the field of novel MNK inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0442] Example 1: 7'-((6-aminopyrimidin-4-yl)amino)-5'-methylspiro[cyclohexane-1,2'-pyrido[2,1-f][1,2 ,4]Triazine]-4',8'(1'H,3'H)-dione hydrochloride (compound 1-1) synthesis
[0443]
[0444] Step 1: Intermediate 1.1 (100 mg, 0.3 mmol), tert-butyl 6-aminopyrimidine-4-carbamate (77.3 mg, 0.37 mmol), Pd 2 (dba) 3 (15mg), Xantphos (15mg), Cs 2 CO 3 (200 mg, 0.61 mmol) and 1,4-dioxane (2.5 mL) were replaced with nitrogen three times, and then the reaction system was heated to 85° C. and stirred for 3 hours. The reaction solution was concentrated under reduced pressure, the residue was slurried with ethanol (2.5mL), filtered, the filter cake was rinsed with a small amount of cold ethanol, and the filter cake was vacuum-dried to obtain (6-((5'-methyl-4',8' -Dicarbonyl-1',3',4',8'-tetrahydrospiro[cyclohexane-1,2'-pyridine[2,1-f][1,2,4]triazine]-7' -yl)amino)pyrimidin-4-yl)tert-butyl carbamate (200 mg, crude) as a yellow solid. m / z:[M+H] + 456.0.
[0445] Step 2: Under ice...
Embodiment 2
[0446] Example 2: 7'-((7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)-5'-methylspiro[cyclohexane-1,2'-pyrido[2, Synthesis of 1-f][1,2,4]triazine]-4',8'(1'H,3'H)-dione hydrochloride (compound 1-2)
[0447]
[0448] Intermediate 1.1 (50mg, 0.15mmol), 7H-pyrrolo[2,3-d]pyrimidin-4-amine (25mg, 0.18mmol), Pd 2 (dba) 3 (10mg), Xantphos (10mg), Cs 2 CO 3 (100 mg, 0.3 mmol) and 1,4-dioxane (1.2 mL) were replaced with nitrogen three times, and then the reaction system was heated to 95° C. and stirred for 6 hours. The reaction solution was concentrated under reduced pressure, and the residue was purified by prep-HPLC (separation condition 2) to obtain compound 1-2 (20 mg, yield: 34%) as a yellow solid. m / z:[M+H] + 380.0; 1 H NMR (400MHz, DMSO-d 6 ):δ12.42(s,1H),9.75(br.s,1H),9.03(s,1H),8.45(s,1H),8.21(s,1H),7.46(s,1H),6.89( s,1H),2.49(s,3H),1.75-1.25(m,10H).
Embodiment 3
[0449] Example 3: 7'-((7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)-5'-chlorospiro[cyclohexane-1,2'-pyrido[2,1 Synthesis of -f][1,2,4]triazine]-4',8'(1'H,3'H)-dione hydrochloride (compound 1-3)
[0450]
[0451] Using the synthesis method of compound 1-2, intermediate 1.1 was replaced by 1.2 to obtain compound 1-3 as a white solid. m / z:[M+H] + 400.0; 1 H NMR (400MHz, DMSO-d 6 ):δ12.27(s,1H),9.25(br.s,1H),9.12(s,1H),8.64(s,1H),8.51(s,1H),7.44(s,1H),6.89( s,1H),1.84-1.16(m,10H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com