glp-1 agonistic polypeptide compound and its salt, synthesis method and application
A GLP-1, 1. GLP-1 technology, applied in the preparation method of peptides, chemical instruments and methods, peptide/protein components, etc., can solve the problems of inability to achieve blood sugar control and weight loss, singleness, limitation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
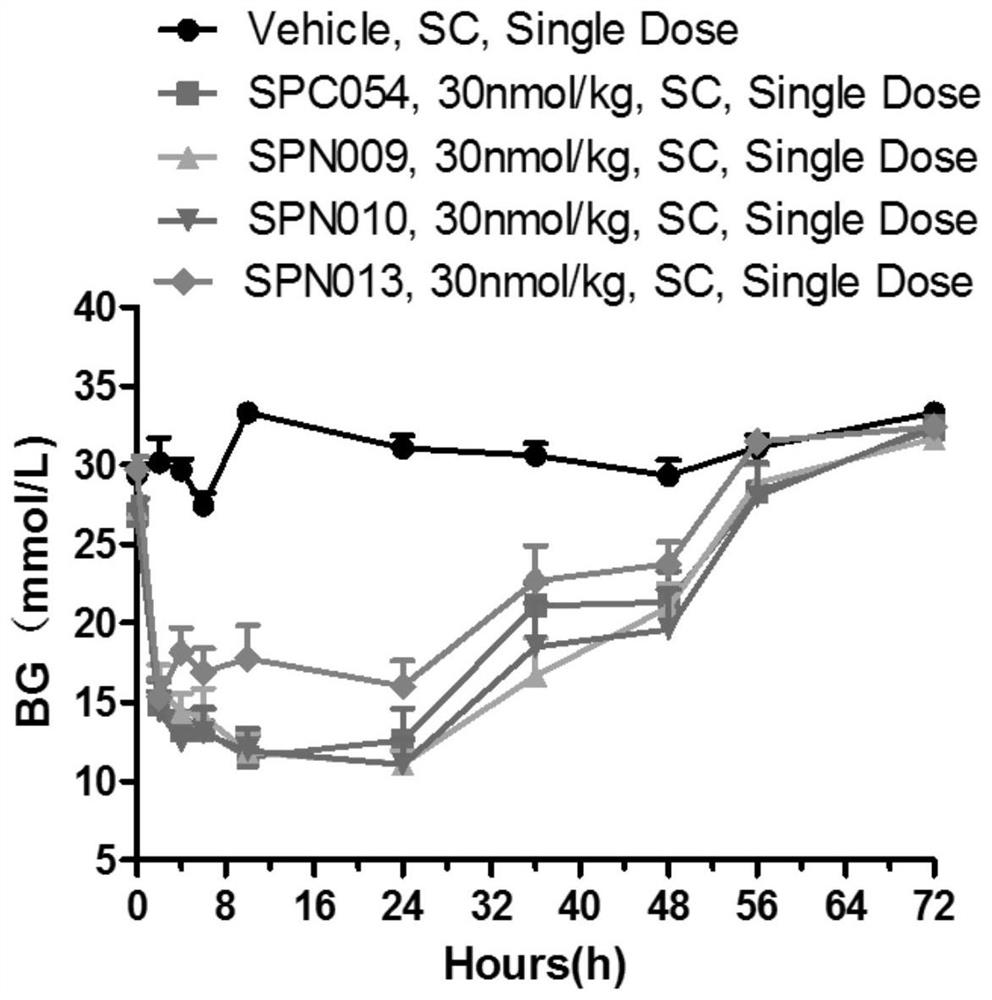
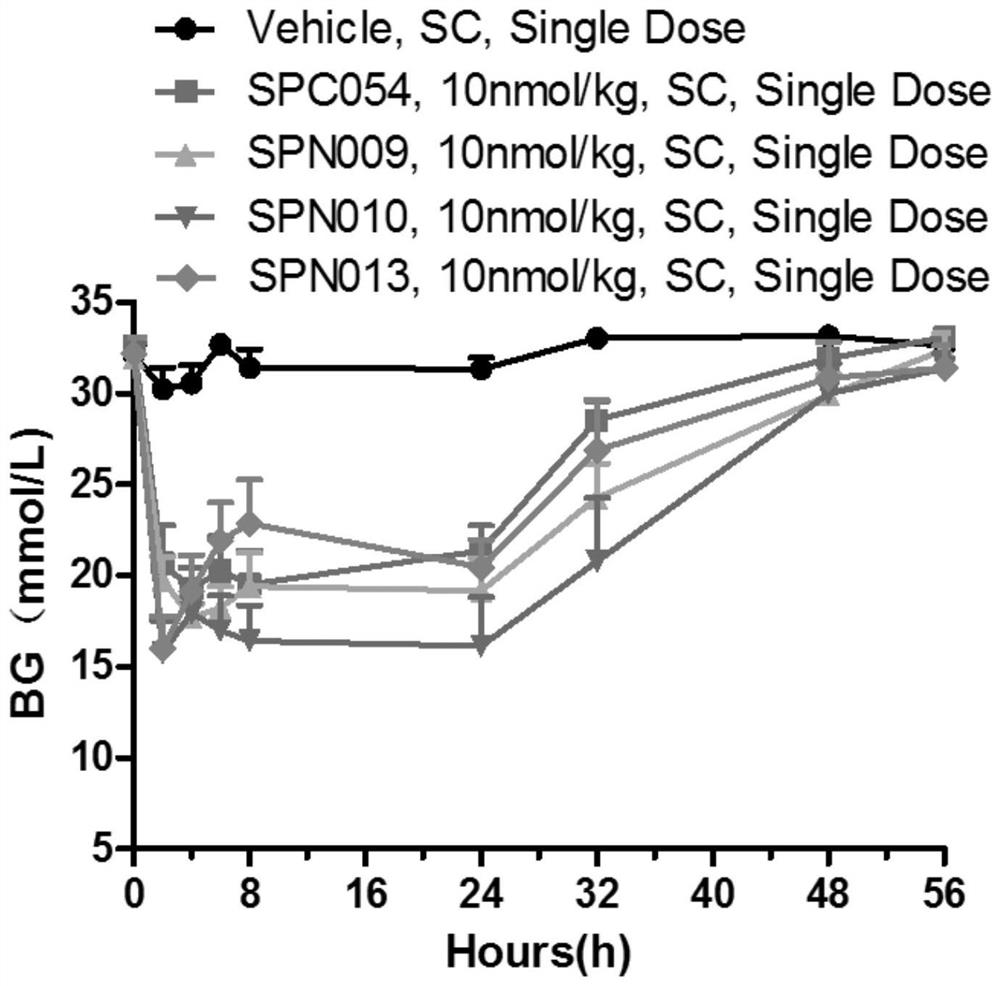
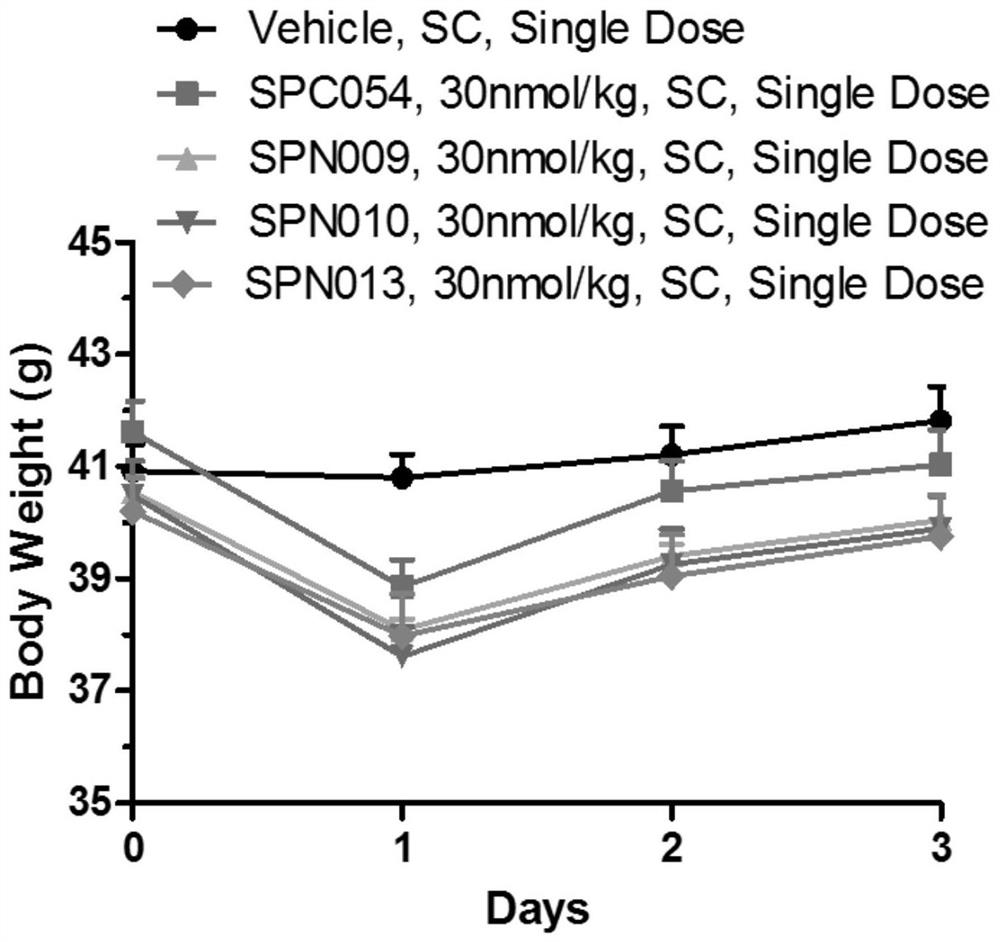
Image
Examples
Embodiment 1
[0124] Embodiment 1, the synthesis of SEQ ID NO: 1 polypeptide compound:
[0125] HX 2 EGTFTSDVSSYLEX 2 QAAK(HOOC-(CH 2 ) 16 -CO-γ-Glu-AEEA-AEEA)EFIAWLVRGRG
[0126] 1. Synthesis of the peptide chain of the polypeptide compound of SEQ ID NO: 1
[0127] 1.1 Resin swelling
[0128] Weigh 10g of Wang-Resin (degree of substitution 0.53mmol / g), swell with 100mL of DCM for 30min, filter to remove DCM, then swell with 100mL of DMF for 30min, rinse with DMF and 100mL of DCM respectively.
[0129] 1.2 Synthesis of Fmoc-Gly-Wang-Resin
[0130] Dissolve Fmoc-Gly(tBu)-OH (15mmol), HOBT (18mmol) and DIC (18mmol) in 100mL of DMF, then add this solution to the resin obtained in the previous step to react for 2 hours, and filter the reaction solution after completion. The resin was washed 3 times with 100 mL each of DCM and DMF.
[0131] 1.3 Removal of Fmoc protecting group
[0132] Add 25% piperidine / DMF (V / V) solution containing 0.1M HOBt to the washed resin to remove Fmoc, and was...
Embodiment 2
[0141] Embodiment 2, the synthesis of SEQ ID NO:2 polypeptide compound:
[0142] HX 2 EGTFTSDVSSYLEX 1 QAAK(HOOC-(CH 2 ) 16 -CO-γ-Glu-AEEA-AEEA)EFIAWLVRGRG
[0143] The synthesis method was the same as in Example 1, and the purified sample solution was collected and freeze-dried to obtain 3.93 g of pure product.
Embodiment 3
[0144] Embodiment 3, the synthesis of SEQ ID NO:3 polypeptide compound:
[0145] HX 3 EGTFTSDVSSYLEX 3 QAAK(HOOC-(CH 2 ) 16 -CO-γ-Glu-AEEA-AEEA)EFIAWLVRGRG
[0146] The synthesis method was the same as in Example 1, and the purified sample solution was collected and freeze-dried to obtain 2.87 g of pure product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com