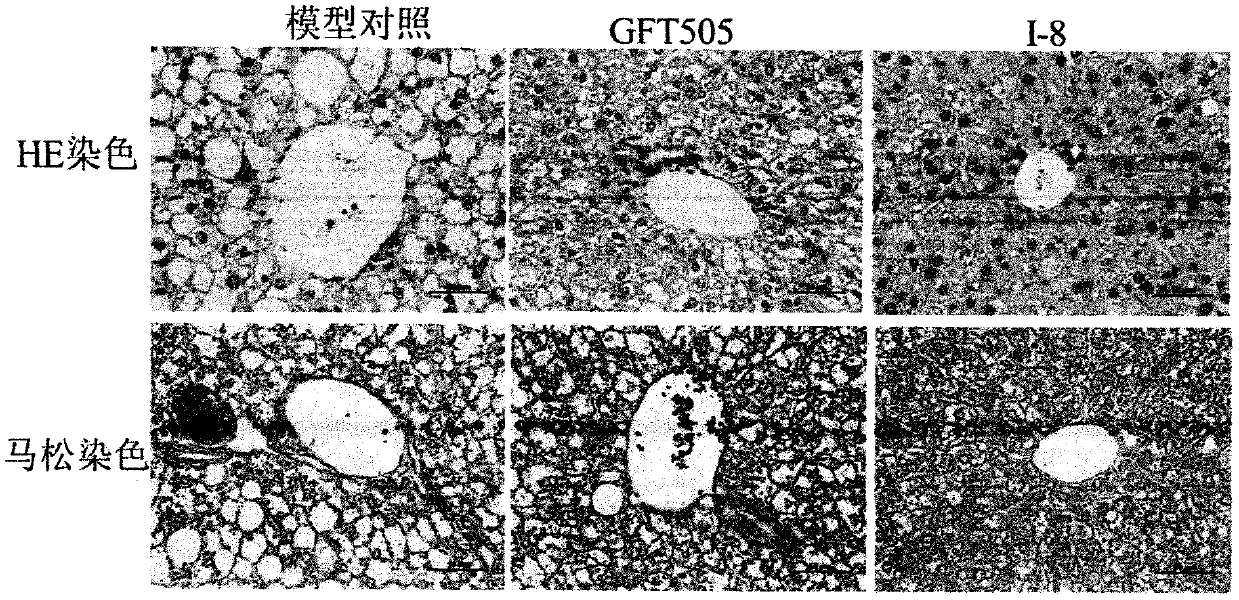
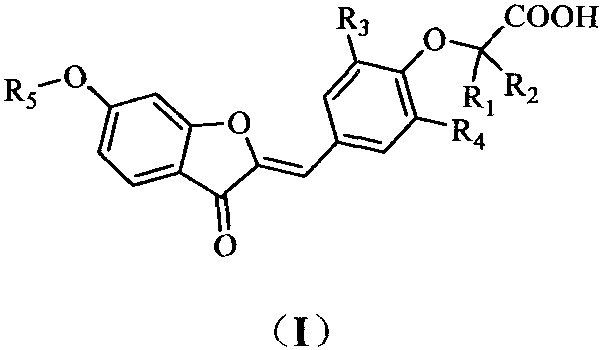
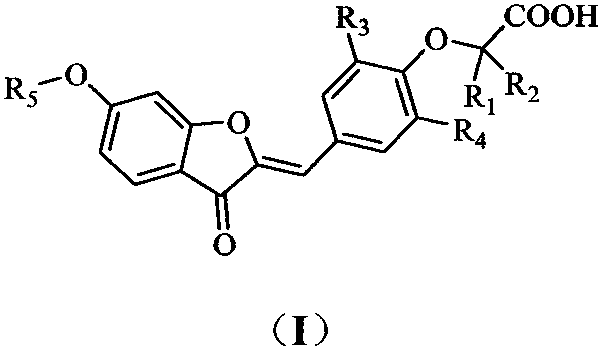
PPARgamma/delta dual agonist, preparation method thereof and application of PPARgamma/delta dual agonist as medicine
A compound and alkyl technology, applied in the field of medicine, can solve the problems of PPARγ/δ dual agonists that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] (Z)-2-(4-((6-methoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)-2,6-dimethylphenoxy)acetic acid ( I-1)
[0087]
[0088] 6-Methoxy-3-benzofuranone 1a (1.0g, 6.1mmol) and methyl 2-(4-formyl-2,6-dimethylphenoxy)acetate 2a (1.3g, 6.1mmol ) was dissolved in ethanol / DMF (20mL, 1:1, v / v) mixed solvent, and 2.3mL of 50% KOH aqueous solution was added dropwise at room temperature, and the solution was reacted at room temperature for 4 hours. After the reaction was detected by TLC, the reaction solution was poured into 30mL of water , adjusted the pH to 1-2 with concentrated hydrochloric acid under vigorous stirring, suction filtered after cooling, washed the filter cake with a small amount of cold water, recrystallized with methanol / DMF (1:1, v / v) to obtain 1.2 g of light yellow crystals, and the yield was 56 %.
[0089] 1 H NMR (300MHz, DMSO-d 6 )δ: 7.75-7.62(m, 3H), 7.13(d, J=1.9Hz, 1H), 6.86, 6.83(dd, J=8.6, 2.0Hz, 1H), 6.69(s, 1H), 4.45(s , 2H), 3.94(s, 3H), 2.31(s, 6H)....
Embodiment 2
[0091] (Z)-2-(4-((6-ethoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)-2,6-dimethylphenoxy)acetic acid ( I-2)
[0092]
[0093] Referring to the preparation method of I-1, 0.64 g of light yellow crystals was obtained with a yield of 50%.
[0094] 1 H NMR (300MHz, DMSO-d 6 )δ: 7.76-7.62(m, 3H), 7.08(s, 1H), 6.81(d, J=8.5Hz, 1H), 6.68(s, 1H), 4.45(s, 2H), 4.22(q, J =6.8Hz, 2H), 2.31(s, 6H), 1.39(t, J=6.8Hz, 3H). 13 C NMR (75MHz, DMSO-d 6 )δ: 181.97, 170.40, 168.41, 167.02, 157.19, 147.18, 132.37, 131.50, 128.20, 114.31, 112.32, 109.64, 100.35, 69.37, 65.04, 16.61, 14.78. - .Anal.calcd.For C 21 h 20 o 6 : C, 68.47; H, 5.47; Found: C, 68.62; H, 5.31.
Embodiment 3
[0096] (Z)-2-(4-((6-propoxy-3-oxobenzofuran-2(3H)-ylidene)methyl)-2,6-dimethylphenoxy)acetic acid ( I-3)
[0097]
[0098] Referring to the preparation method of I-1, 0.49 g of light yellow crystals was obtained with a yield of 35%.
[0099] 1 H NMR (300MHz, DMSO-d 6 )δ: 7.76-7.63(m, 3H), 7.11(d, J=1.6Hz, 1H), 6.84, 6.81(dd, J=8.5, 1.8Hz, 1H), 6.69(s, 1H), 4.45(s , 2H), 4.12(t, J=6.5Hz, 2H), 2.31(s, 6H), 1.85-1.73(m, 2H), 1.01(t, J=7.4Hz, 3H). 13 C NMR (75MHz, DMSO-d 6 )δ: 181.93, 170.42, 167.20, 162.52, 157.15, 147.20, 132.37, 131.53, 128.32, 114.31, 112.32, 109.64, 99.99, 70.77, 65.04, 22.25, 16.63, 10.18. - .Anal.calcd.For C 22 h 22 o 6 : C, 69.10; H, 5.80; Found: C, 69.35; H, 5.71.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com