Cyclization preparation method of compounds related with polymyxin B
A polymyxin and compound technology, which is applied in the field of cyclization preparation of polymyxin B related compounds, can solve the problems of unsuitability for large-scale production, low yield, poor stability of polymyxin B, and the like, and achieves good medicine. The effect of using prospects, reducing costs, and improving yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
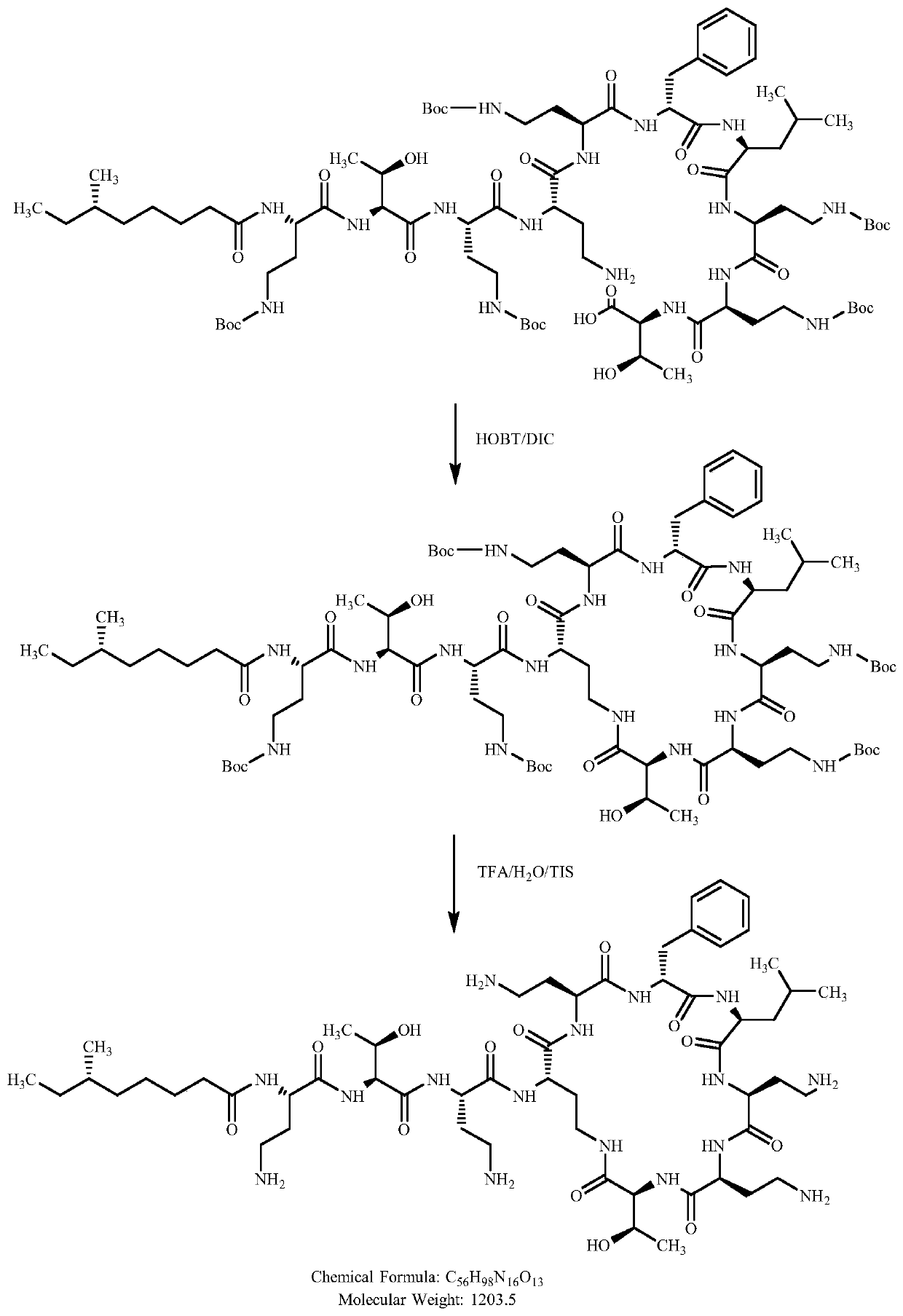
[0034] The cyclization preparation method of polymyxin B1, the synthesis process is as follows figure 1 As shown, it specifically includes the following steps:
[0035] step one:
[0036] CH 3 CH 2 (CH 3 )CH(CH 2 ) 4 Synthesis of CO-Dab(Boc)-Thr(tBu)-Dab(Boc)-Dab-Dab(Boc)-D-Phe-Leu-Dab(Boc)-Dab(Boc)-Thr(tBu)-OH:
[0037] 1) Add 10 g of CTC-Resin with a degree of substitution of 0.4 to 0.6 mmol / g into the polypeptide reactor, add 2 g of Fmoc-Thr(tBu)-OH and 10 ml of DIEA to react for 2 hours, remove the reaction liquid, and wash twice with DMF; After removing the Fmoc protection, wash twice with DMF, once with DCM, and twice with DMF;
[0038] 2) Then Fmoc--Dab(Boc)-OH, Fmoc-Dab(Boc)-OH, Fmoc-Leu-OH, Fmoc-D-Phe-OH, Fmoc-Dab(Boc)-OH, Fmoc-Dab- OH, Fmoc-Dab(Boc)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Dab(Boc)-OH, Fmoc-Dab(Boc)-OH, CH 3 CH 2 (CH 3 )CH(CH 2 ) 4 COOH, the reaction time is 2 hours, the deprotection time is 0.5 hours, and the end point of the reaction is determined b...
Embodiment 2
[0057] The cyclization preparation method of polymyxin B1 comprises the following steps:
[0058] step one:
[0059] CH3CH2(CH3)CH(CH2)4CO-Dab(Boc)-Thr(tBu)-Dab(Boc)-Dab-Dab(Boc)-D-Phe-Leu-Dab(Boc)-Dab(Boc)-Thr( Synthesis of tBu)-OH:
[0060] 1) Add 10 g of CTC-Resin with a degree of substitution of 0.4 to 0.6 mmol / g into the polypeptide reactor, add 2 g of Fmoc-Thr(tBu)-OH and 10 ml of DIEA to react for 2 hours, remove the reaction solution, and wash twice with DMF; After removing the Fmoc protection, wash twice with DMF, once with DCM, and twice with DMF;
[0061] 2) Then Fmoc--Dab(Boc)-OH, Fmoc-Dab(Boc)-OH, Fmoc-Leu-OH, Fmoc-D-Phe-OH, Fmoc-Dab(Boc)-OH, Fmoc-Dab- OH, Fmoc-Dab(Boc)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Dab(Boc)-OH, Fmoc-Dab(Boc)-OH, CH3CH2(CH3)CH(CH2)4COOH, reaction time Both are 2 hours, the deprotection time is 0.5 hours, and the reaction end point is determined by the ninhydrin method; after the reaction, wash twice with DMF, once with DCM, and twice with DMF;
...
Embodiment 3
[0080] The cyclization preparation method of polymyxin B1 comprises the following steps:
[0081] step one:
[0082] CH3CH2(CH3)CH(CH2)4CO-Dab(Boc)-Thr(tBu)-Dab(Boc)-Dab-Dab(Boc)-D-Phe-Leu-Dab(Boc)-Dab(Boc)-Thr( Synthesis of tBu)-OH:
[0083] 1) Add 10 g of CTC-Resin with a degree of substitution of 0.4 to 0.6 mmol / g into the polypeptide reactor, add 2 g of Fmoc-Thr(tBu)-OH and 10 ml of DIEA to react for 2 hours, remove the reaction solution, and wash twice with DMF; After removing Fmoc protection, wash twice with DMF, once with DCM, and twice with DMF
[0084] 2) Then Fmoc--Dab(Boc)-OH, Fmoc-Dab(Boc)-OH, Fmoc-Leu-OH, Fmoc-D-Phe-OH, Fmoc-Dab(Boc)-OH, Fmoc-Dab- OH, Fmoc-Dab(Boc)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Dab(Boc)-OH, Fmoc-Dab(Boc)-OH, CH3CH2(CH3)CH(CH2)4COOH, reaction time Both are 2 hours, the deprotection time is 0.5 hours, and the reaction end point is determined by the ninhydrin method; after the reaction, wash twice with DMF, once with DCM, and twice with DMF;
[0085]...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap