Methods for treating muscular dystrophy
A Duchenne muscular dystrophy, poor technology, used in gene therapy, biochemical equipment and methods, pharmaceutical formulations, etc., to solve problems such as limited pharmacological options
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
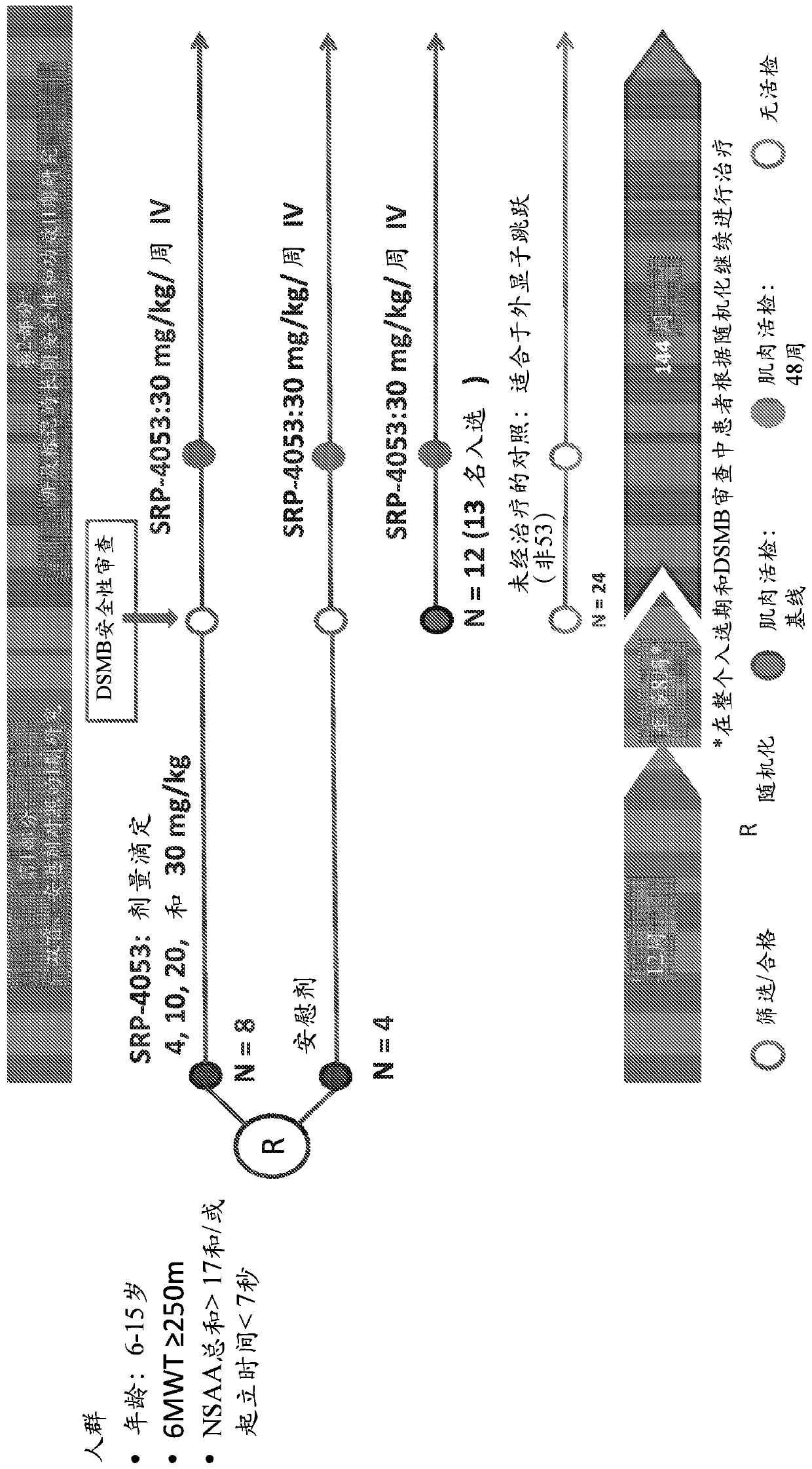
[0195] All examples are derived from the following ongoing first-in-human clinical trials testing the safety and efficacy of SRP-4053. The results reported here were obtained at week 48 during part 2 of the study.
[0196] Phase I / II Study of SRP-4053 in DMD Patients
[0197] ClinicalTrials.gov Identifier: NCT02310906
[0198] This is the first human multiple-dose 2-part study evaluating the safety, tolerability, efficacy and pharmacokinetics of SRP-4053 in Duchenne muscular dystrophy (DMD) patients with deletions suitable for exon 53 skipping .
[0199] Study Type: Interventional
[0200] Study Design: Allocation: Randomized
[0201] Intervention Mode: Parallel Assignment
[0202] Blinding: Quadruple (Participant, Care Provider, Investigator, Outcome Assessor)
[0203] Primary Purpose: Healing
[0204] Official Title: 2-Part, Randomized, Double-Blind, Placebo-Controlled, Dose-Titration, Safety, Tolerability, and Pharmacokinetic Study (Part 1), followed by SRP-4053 in E...
example 1
[0257] Example 1: Biochemical Efficacy Assessment
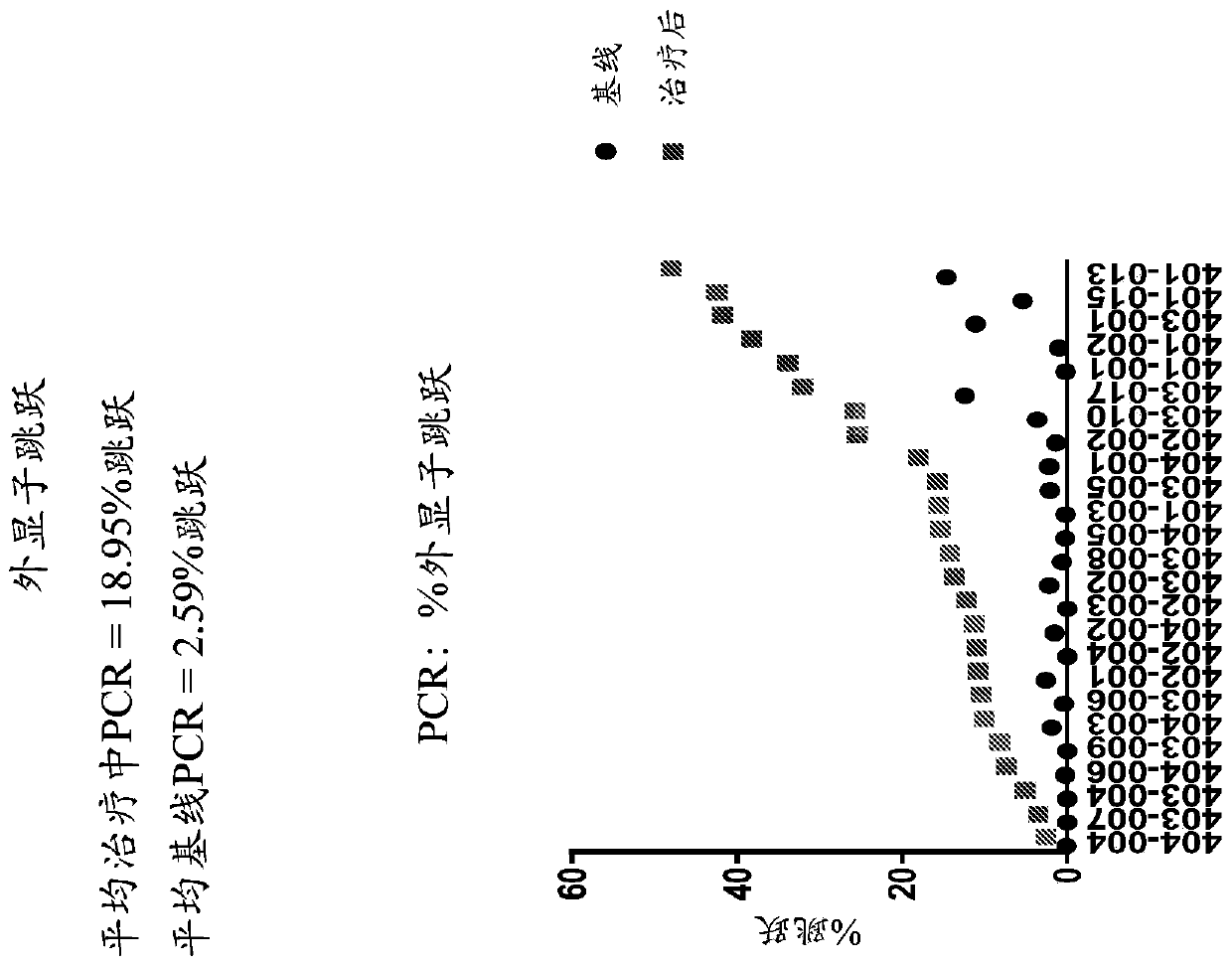
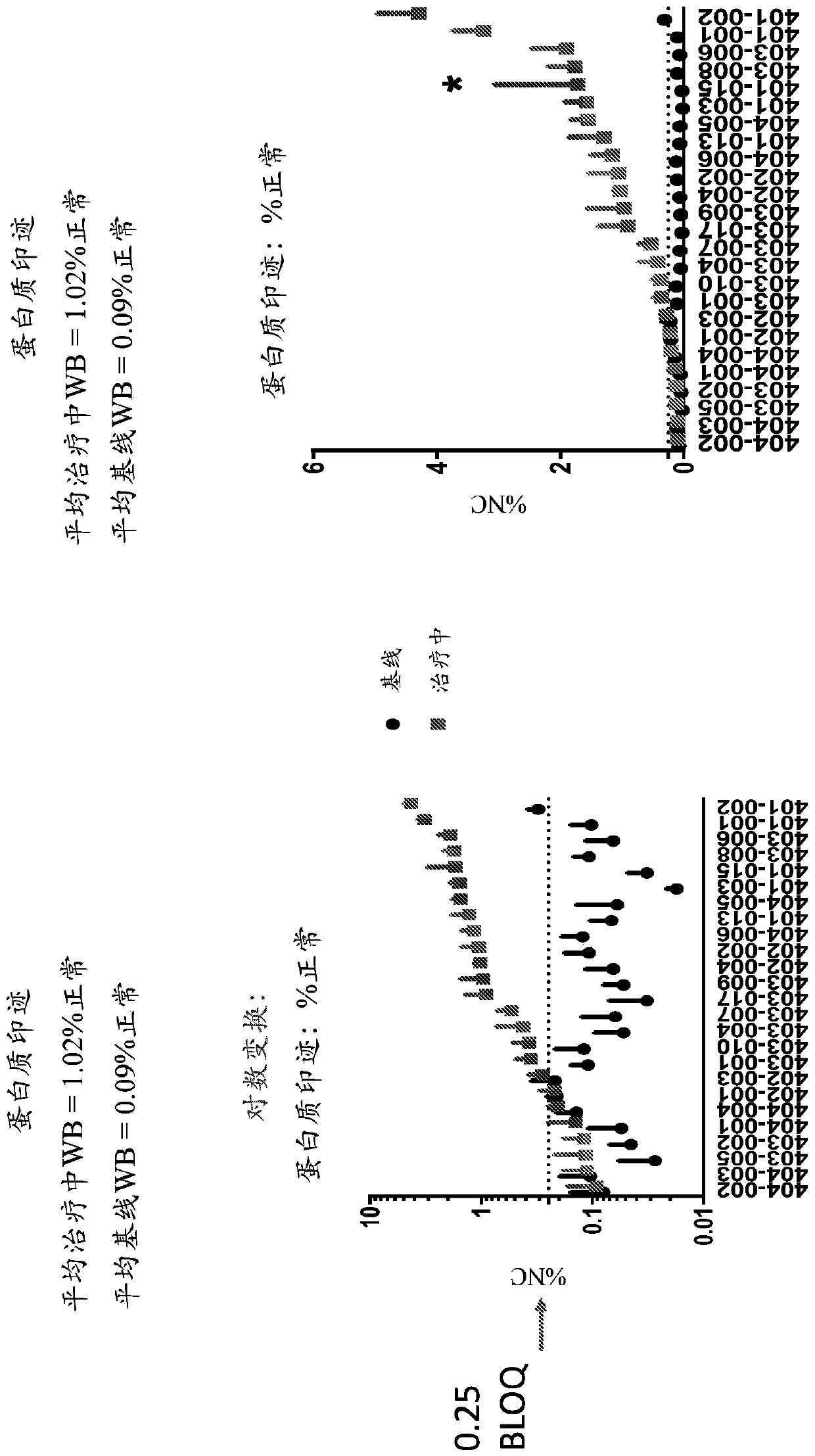
[0258] Paired muscle biopsies of the biceps brachii at baseline and on treatment were obtained from 25 patients participating in a multisite first-in-human trial evaluating the safety, tolerability, and tolerability of 30 mg / kg SRP-4053 administered weekly by intravenous infusion and dystrophin production (ClinicalTrials.gov Identifier: NCT02310906). For each surgery, two pieces of muscle are removed: the A block and the B block. For all assays, A and B blocks were analyzed separately.
[0259] Muscle biopsies were tested by optimized methods to assess dystrophin protein quantity (Western blot, primary biological endpoint) and exon skipping (RT-PCR). Novel automated imaging analysis (MuscleMap TM )) Immunohistochemistry was used to assess dystrophin localization (mean fiber intensity).
[0260] For Western blot analysis: Panels A and B run on duplicate gels = 4 tests averaged
[0261] For RT-PCR analysis: Blocks A and B ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com