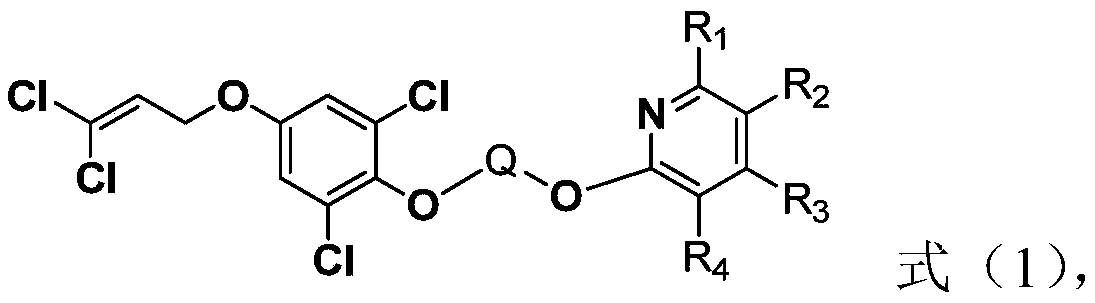
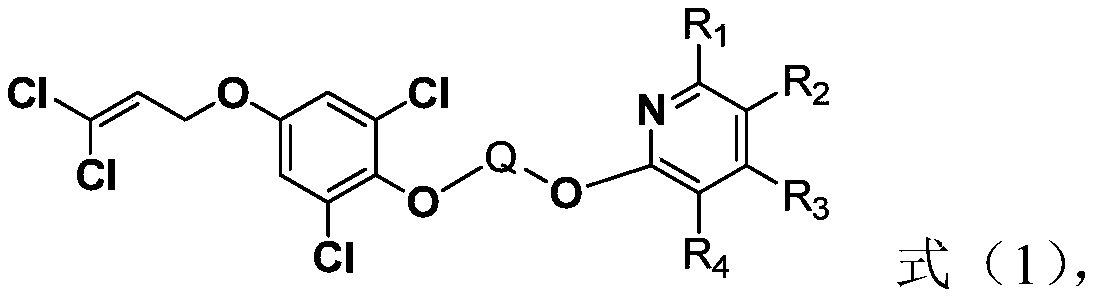
Cyanopyridine-containing dichloropropene ether compound, preparation method and application thereof, and insecticide
A technology of dichloropropene ethers and cyanopyridines, applied to ether compounds and the aforementioned cyano group-containing fields, can solve problems such as poor insecticidal effect, achieve excellent insecticidal effect, excellent pest control effect, and broad-spectrum Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0069] Step A: Preparation of 4-(3,3-dichloroallyloxy)phenol
[0070] Dissolve hydroquinone (900mmol) in 300mL anhydrous acetone, add anhydrous potassium carbonate (450mmol) in batches, mechanically stir and heat to reflux temperature, then slowly add 1,1,3-trichloropropene (300mmol) ) 100mL of acetone solution, after the addition is complete, continue to react at reflux temperature, and TLC detects that the reaction is complete after about 8 hours. After the reaction solution is cooled, potassium carbonate is filtered out, and the filtrate is directly added with silica gel to mix the sample, and the product obtained by column chromatography is 42.7 g of brown solid, with a yield of 65%.
[0071] Step B: Preparation of 2,6-dichloro-4-(3,3-dichloroallyloxy)phenol
[0072] To the 200mL toluene solution with 4-(3,3-dichloroallyloxy)phenol (182.6mmol) dissolved in 200mL, add 0.4g of diethylamine, configure the condenser reflux tube and exhaust gas absorption device, stir and heat to At...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com