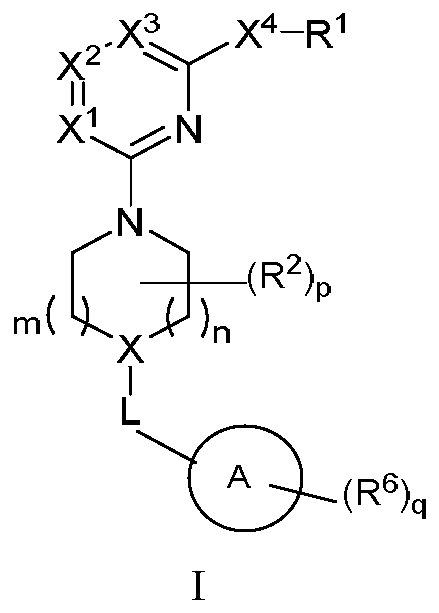
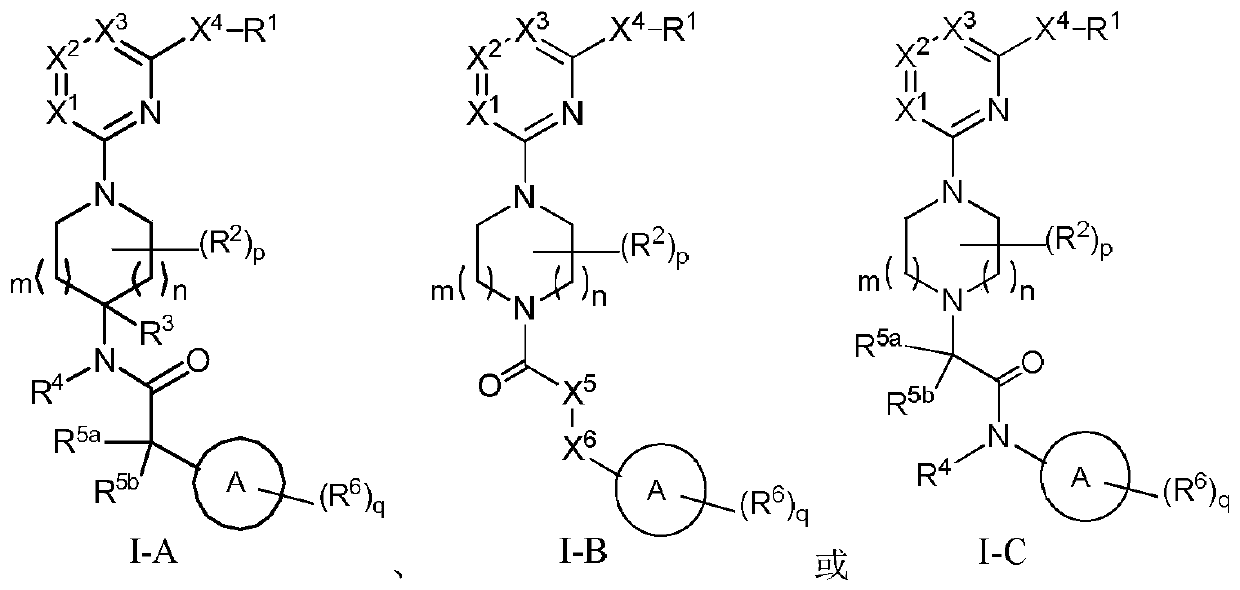
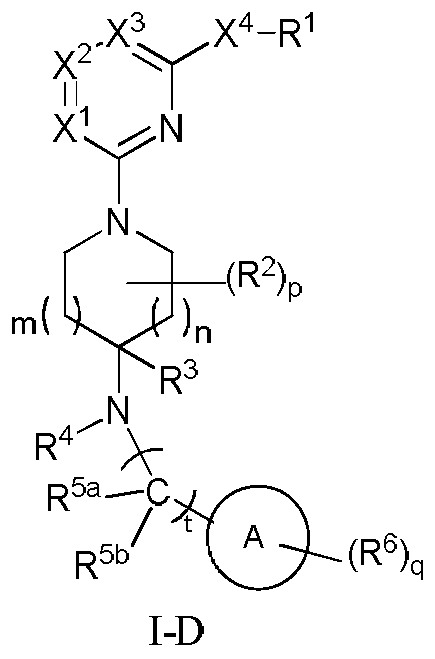
Nitrogen heterocyclic compound, pharmaceutical composition containing nitrogen heterocyclic compound, and preparation method and application of nitrogen heterocyclic compound
A compound and mixture technology, applied in the field of new nitrogen heterocyclic compounds, can solve problems such as the listing of inhibitors, and achieve good inhibitory effect and good pharmacokinetic effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0410] Example 1: 2-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)-N-(1-(4-methyl-6-((5-methyl- 1H-pyrazol-3-yl)amino)pyrimidin-2-yl)piperidin-4-yl)acetamide (compound 1)
[0411]
[0412] The first step: 2-(6-iodopyridin-3-yl) methyl acetate (1b)
[0413] Compound 1a (2g, 10.78mmol), acetyl chloride (1.28g, 16.16mmol), and sodium iodide (20.04g, 107.75mmol) were added to acetonitrile (50mL), heated to 80°C for 16h, and cooled to room temperature after the reaction . The reaction solution was concentrated to dryness to obtain compound 1b (2.8 g).
[0414] MS (ESI, m / z): 278.0 [M+H] + .
[0415] The second step: methyl 2-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)acetate (1d)
[0416] Compound 1b (0.4g, 1.44mmol), 4-fluoropyrazole (186.39mg, 2.17mmol), salicylaldoxime 1c (39.60mg, 288.75μmol), cuprous oxide (20.6mg, 144μmol) and cesium carbonate (941.32mg , 2.89mmol) was placed in a reaction flask, acetonitrile (10mL) was added, and the reaction was carried out at 80°C for ...
Embodiment 2
[0434] Example 2: 2-(6-(4-fluoro-1H-pyrazol-1-yl)pyridin-3-yl)-N-(1-(4-methyl-6-((5-methyl- 1H-pyrazol-3-yl)amino)pyrimidin-2-yl)piperidin-4-yl)propionamide (Compound 2)
[0435]
[0436] The first step: methyl 2-(6-chloropyridin-3-yl)propionate (2a)
[0437] Compound 1a (2.04g, 10.78mmol) was dissolved in THF (30mL), cooled to -78°C, and 4.74mL of 2.5Mn-BuLi in THF was slowly added under nitrogen protection and stirred for 30min, then MeI (15.61g, 107.75mmol), and then reacted for 12h. After the reaction was completed, it was raised to room temperature, and 100 mL of saturated NH 4 Cl solution, extracted with EA (100 mL x 3). The organic phases were combined and washed with saturated brine, dried over anhydrous sodium sulfate, and then separated and purified by silica gel column chromatography (PE:EA=10:1-1:1) to obtain compound 2a (1.1 g).
[0438] MS m / z(ESI): 200.1[M+H] + .
[0439] The second step: methyl 2-(6-iodopyridin-3-yl)propionate (2b)
[0440] Compound 2...
Embodiment 3
[0452] Example 3: 2-(6-(3,5-dimethylisoxazol-4-yl)pyridin-3-yl)-N-(1-(4-methyl-6-((5-methyl Base-1H-pyrazol-3-yl)amino)pyrimidin-2-yl)piperidin-4-yl)propionamide (compound 3)
[0453]
[0454] The first step: methyl 2-(6-(3,5-dimethylisoxazol-4-yl)pyridin-3-yl)propionate (3b)
[0455] Compound 2a (200 mg, 901.65 μmol) was dissolved in dioxane (10 mL), and compound 3a (246.29 mg, 1.08 mmol), K 2 CO 3 (253.93 mg, 1.80 mmol) and water (2 mL), and finally Pd(dppf)Cl 2 · DCM (150.15 mg, 180.33 μmol), heated to 90° C. for 4 h under nitrogen protection. After the reaction was completed, it was cooled to room temperature, diluted with 50 mL of water, and extracted with EA (40 mL x 3). The organic phases were combined and washed with saturated brine, dried over anhydrous sodium sulfate, and separated by silica gel column chromatography (DCM:MeOH=50:1-10:1) to obtain compound 3b (185 mg).
[0456] MSm / z(ESI): 261.1[M+H] + .
[0457] The second step: 2-(6-(3,5-dimethylisoxazol-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com