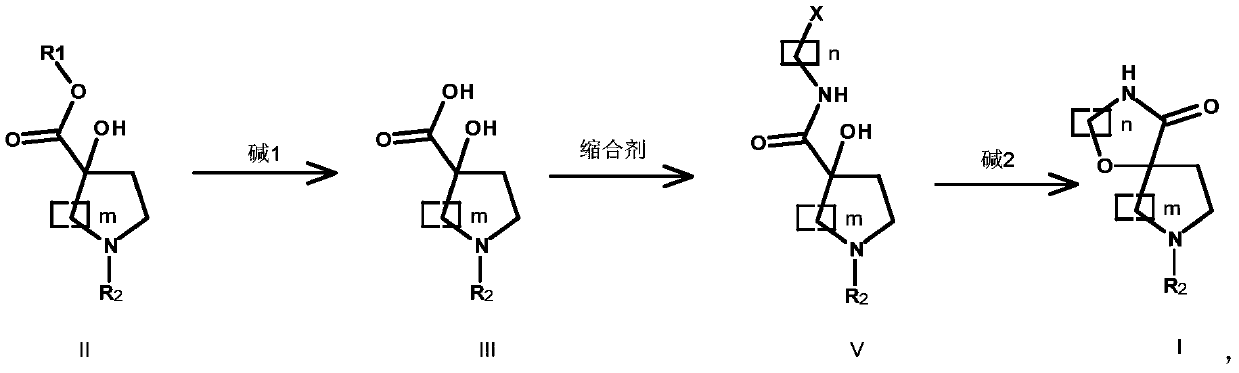
Preparation method of oxazaspiro compound
A compound, benzyloxycarbonyl technology, applied in the field of pharmaceutical intermediate synthesis, can solve the problems of unsuitability for large-scale production, potential safety hazards, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]
[0051] Preparation of compound III-1:
[0052] Compound II-1 (94.9g, 366.4mmol, 1.0eq.) was dissolved in MeOH (700mL), and at 0°C, an aqueous solution (30mL) of NaOH (29.3g, 732.8mmol, 2.0eq.) was added dropwise and heated to The reaction was stirred at 60°C for 18h. TLC showed that the reaction of the raw materials was complete, the reaction solution was lowered to room temperature, the reaction solution was concentrated to an oily substance, water was added, EA was extracted twice, the aqueous phase was adjusted to pH 3-4 with saturated citric acid aqueous solution, EA was extracted, the organic phases were combined, and Washed with saturated brine, anhydrous MgSO 4 After drying, filtering and concentrating, compound III-1 was obtained as light yellow solid 83.1 g, yield 92.3%.
[0053] Preparation of compound V-1:
[0054] Compound III-1 (30.0g, 122.3mmol, 1.0eq.) was dissolved in DMF (300mL), cooled to 0°C, and compound IV-1 (2-chloroethylamine hydrochloride) ...
Embodiment 2
[0058]
[0059] Preparation of compound III-2:
[0060] Compound II-2 (90.01g, 292.8mmol, 1.0eq.) was dissolved in EtOH (700mL), at room temperature, an aqueous solution (30mL) of LiOH (7.01g, 292.8mmol, 1.0eq.) was added, heated to reflux and stirred Reaction 18h. TLC showed that the reaction of the raw materials was complete, the reaction solution was lowered to room temperature, the reaction solution was concentrated to an oily substance, water was added, EA was extracted twice, the aqueous phase was adjusted to pH 3-4 with saturated citric acid aqueous solution, EA was extracted, the organic phases were combined, and Washed with saturated brine, anhydrous MgSO 4 After drying, filtering and concentrating, compound III-2 was obtained as 73.1 g of light yellow solid, with a yield of 89.3%.
[0061] Preparation of Compound V-2:
[0062] Compound III-2 (30.0g, 107.4mmol, 1.0eq.) was dissolved in DMF (300mL), cooled to 0°C, and compound IV-2 (1-chloromethylamine) (14.07g, ...
Embodiment 3
[0066]
[0067] Preparation of compound III-3:
[0068] Compound II-3 (90.1g, 324.5mmol, 1.0eq.) was dissolved in MeOH (700mL), at 0 ℃, an aqueous solution (30mL) of KOH (54.6g, 973.5mmol, 3.0eq.) was added, heated to reflux and stirred Reaction 16h. TLC showed that the reaction of the raw materials was complete, the reaction solution was lowered to room temperature, the reaction solution was concentrated to an oily substance, water was added, EA was extracted twice, the aqueous phase was adjusted to pH 3-4 with saturated citric acid aqueous solution, EA was extracted, the organic phases were combined, and Washed with saturated brine, anhydrous MgSO 4 After drying, filtering and concentrating, Compound III-3 was obtained as 76.23 g of light yellow solid, with a yield of 94.3%.
[0069] Preparation of Compound V-3:
[0070] Compound III-3 (30.0g, 120.4mmol, 1.0eq.) was dissolved in DMF (300mL), cooled to 0°C, and compound IV-1 (2-chloroethylamine hydrochloride) (20.9g, 18...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com