Recombinant virus with codon-pair deoptimized region and uses thereof for the treatment of cancer
A codon and virus technology, applied in the field of recombinant viruses with deoptimized regions of codon pairs and their use in the treatment of cancer, can solve the problem of no effect on short-term or long-term survival of patients, long-term side effects of retroviral therapy, and insertion inducement. change, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0359] Modification of the PV sequence to alter the codon biscore relative to human codon pair bias.
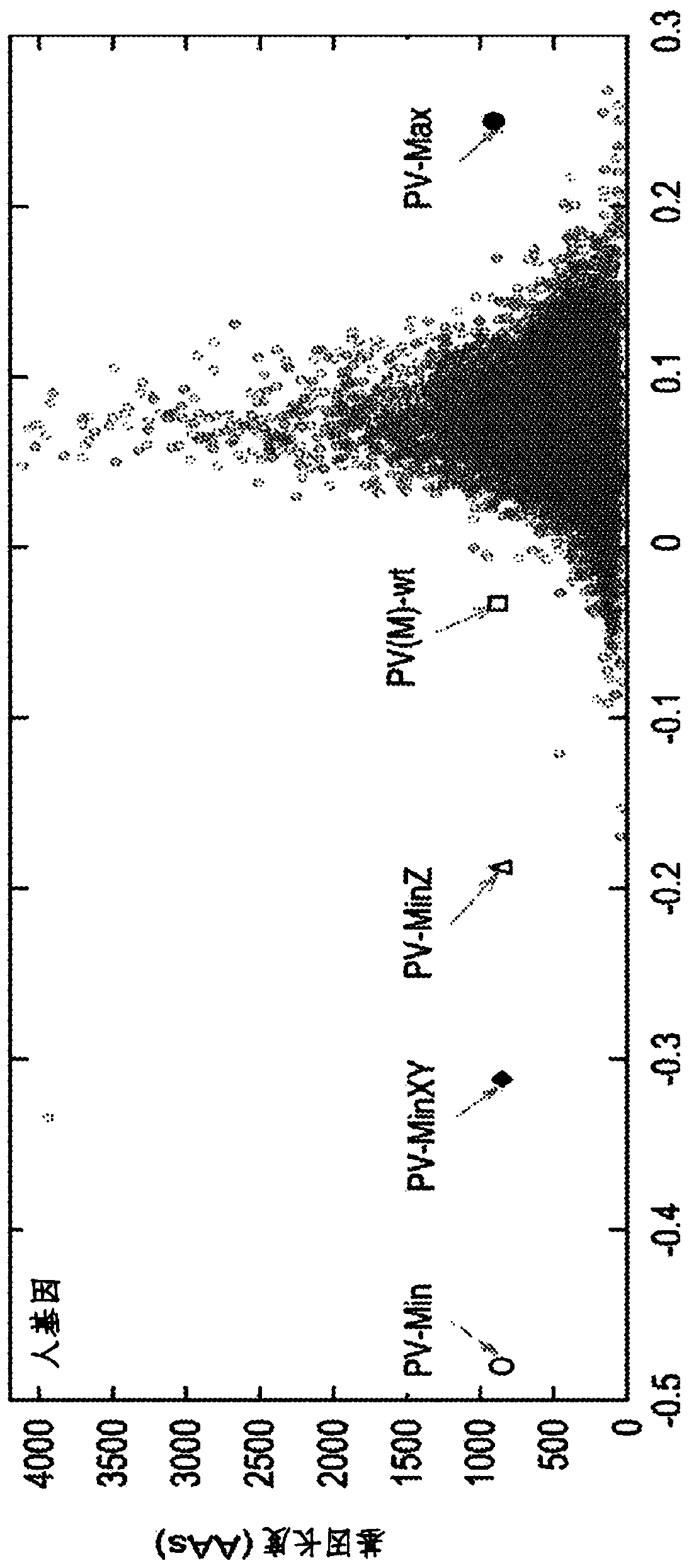
[0360] Our algorithm was given as input the DNA coding sequence of the P1 structural region of poliovirus type 1 Mahoney strain (PV(M)-wt), and the CPB of this region was modified ( figure 2 ). After shuffling using simulated annealing, we generated two new fragments with shuffled codons and thus new codon pairs. The first was designed as PV-Max with a positive CPB of +0.246. This positive CPB indicates that PV-Max uses the overrepresented codon pairs on average. The second was designed as PV-Min with a negative CPB of -0.474. This negative CPB indicates that PV-Min utilizes on average the underrepresented codon pairs ( figure 1 ). The first four codons (12 nucleotides) of PV-Min and PV-Max were unchanged and remained the same as wild-type poliovirus codons. This is done so that translations can be started equally across all entities. Likewise, the P1 region was chos...
Embodiment 2
[0363] Construction of codon pair deoptimized poliovirus
[0364] Plasmids containing the cDNA of synthetic recombinant viruses of the above genotypes or any other variants are amplified, purified and linearized by digestion with the restriction endonuclease FspI (which cleaves within the vector sequence). The resulting linearized cDNA (containing the recognition motif for DNA-dependent RNA polymerase T7 prior to the 5' insertion site of the viral cDNA) was used for in vitro transcription using T7 polymerase to generate full-length viral RNA. The resulting viral RNA was used to transfect HeLa cells by the Dextran-sulfate method to generate infectious virus. Cytopathic effect of transfected cells was observed, indicating productive modified virus infection, and infectious virus was propagated in HeLa cells, purified and frozen for indefinite storage.
Embodiment 3
[0366] The modified virus was attenuated compared to the wild-type virus, but it grew and killed adenocarcinoma cervical cancer HeLa R19 cells.
[0367] After transfection of five PV-Min-derived RNAs into HeLa R19 cells, each product virus was passaged twice to ensure that the viruses were properly amplified, since RNA transfection was not as efficient as natural infection by the virus itself. Next, plaque analysis was performed to clarify the apparent PFU / ml titer (apparent PFU / ml titer) of each virus ( image 3 ). These subclones produced viruses with varying degrees of attenuation. Viruses containing P1 fragments X and Y were each attenuated by 0.8-1 log 10 ; however, when added together, they produced the virus PV-MinXY, which was significantly attenuated by 2.5 orders of magnitude. Virus PV-MinZ is also attenuated by 2.5log like PV-MinXY 10about. Thus, construct PV-YZ failed to produce viable virus when the Y segment was returned to PV-MinZ ( image 3 ). These vary...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com