Compounds for separation of rare earth elements and s-, p-, d- metals, method of separation, and use thereof
A rare earth element, compound technology, applied in the field of p-block, separation of rare earth elements and/or s-block, d-block metal compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
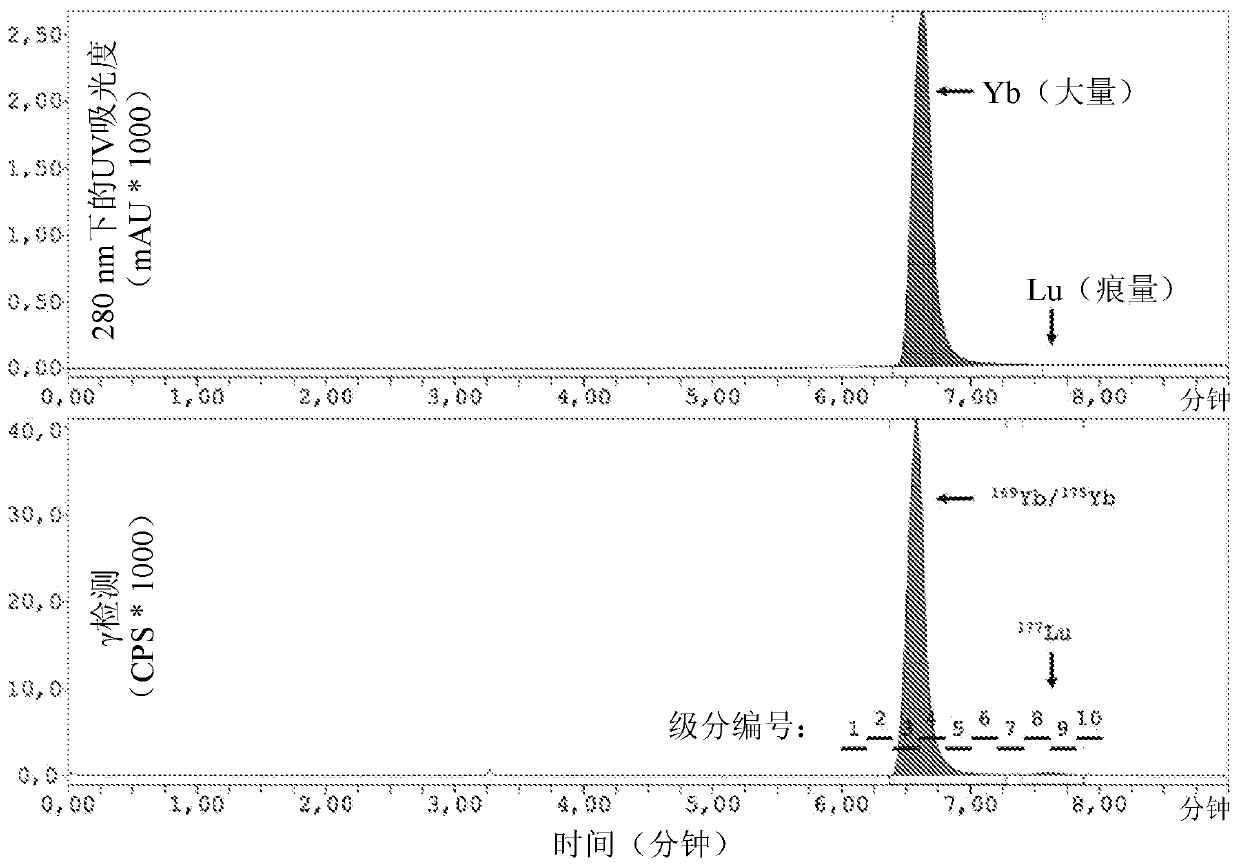
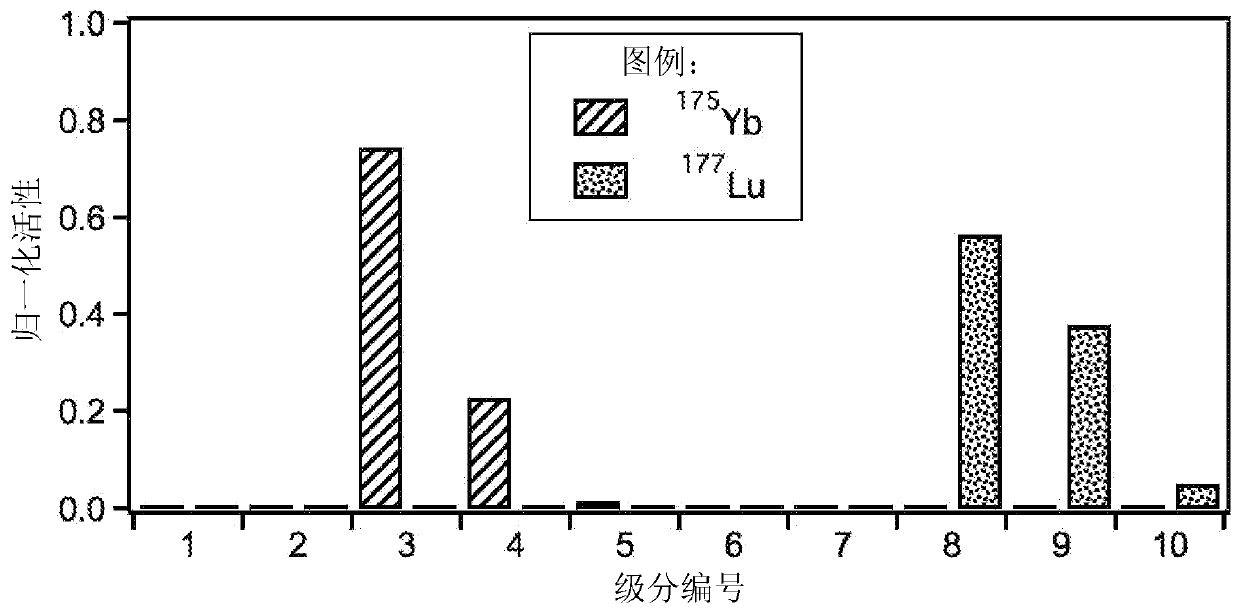
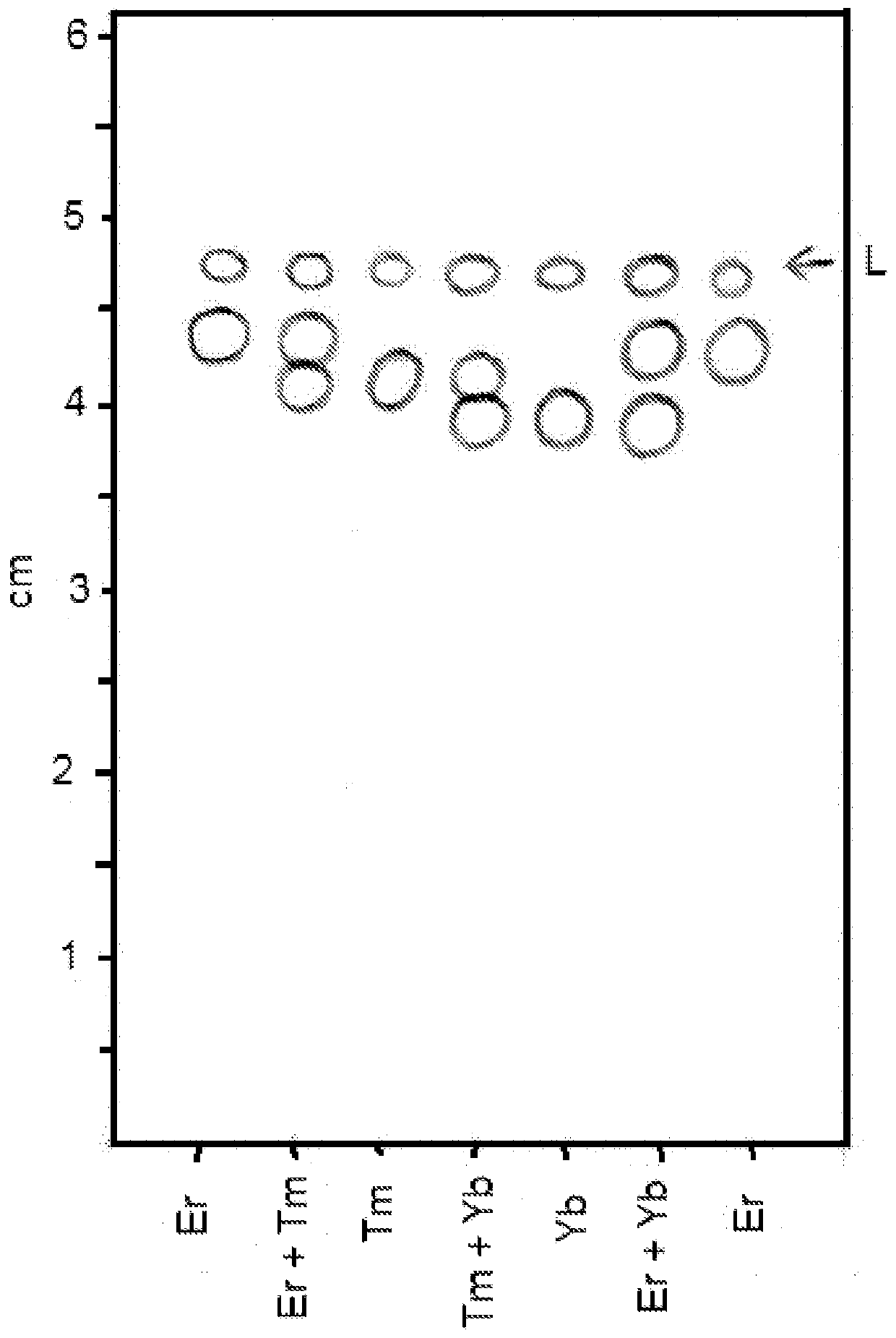
Image
Examples
example 1
[0126] Example 1: 2,2',2"-(10-((6-fluoropyridin-2-yl)methyl)-1,4,7,10-tetraazacyclododecane-1,4,7 - Preparation of triyl) triacetic acid (1)
[0127] And the filtrate was concentrated on a rotary evaporator. The resulting oil was purified on preparative HPLC (C18 column, acetonitrile / water gradient with 0.1% trifluoroacetic acid in the mobile phase). Fractions containing pure product as tert-butyl ester were combined, evaporated and dried under high vacuum. The residue was dissolved in neat trifluoroacetic acid (3 mL) and stirred at room temperature for 24 hours. Trifluoroacetic acid was evaporated on a rotary evaporator. The residue was dissolved in distilled water (2 ml), loaded onto a solid phase extraction cartridge (C18 reverse phase, 500 mg) and eluted with distilled water (10 mL). The eluate was lyophilized, redissolved in distilled water (2 mL) and lyophilized again to yield 199 mg of product (0.283 mmol, 84% yield relative to B) as a white fluffy solid.
[0128...
example 2
[0131] Example 2: 2,2',2"-(10-((6-chloropyridin-2-yl)methyl)-1,4,7,10-tetraazacyclododecane-1,4,7 - Preparation of triyl) triacetic acid (2)
[0132] 69% yield relative to 2-(bromomethyl)-6-chloropyridine).
[0133] 1 H NMR (D with internal dioxane reference 2 O,95℃,500MHz):δ H 3.28-3.35 (ring, m, 4H); 3.35-3.42 (ring, m, 4H); 3.51-3.60 (ring, m, 8H); 3.73 (CH 2 –COOH,s,4H); 4.13(CH 2 –COOH,s,2H); 4.56(CH 2 – aromatic, s, 2H); 7.63 (aromatic, d, 1H, 3 J HH =8Hz); 7.64 (aromatic, d, 1H, 3 J HH =8Hz); 8.00(aromatic,t,1H, 3 J HH = 8Hz); 13 C{ 1 H} NMR (D with internal dioxane reference 2 O,95℃,125MHz):δ C 49.7 (ring, s); 49.9 (ring, s); 51.5 (ring, s); 51.9 (ring, s); 54.4 (CH 2 –COOH,s); 55.5(CH 2 –COOH,s); 58.6 (CH 2 – aromatic, s); 124.4 (aromatic, s); 126.1 (aromatic, s); 142.2 (aromatic, s); 151.7 (aromatic, s); 152.2 (aromatic, s); ,s); 172.9(CO,s). HRMS(ESI)m / z:[(M–H) – ](C 20 h 29 ClN 5 o 6 ) calculated value: 470.1812, measured value: 470.1811....
example 3
[0134] Example 3: 2,2',2"-(10-((6-bromopyridin-2-yl)methyl)-1,4,7,10-tetra
[0135] The reaction of produced approximately 179 mg of product (0.232 mmol, 69% yield relative to B) as a white fluffy solid.
[0136] 1 H NMR (D with internal dioxane reference 2 O,95℃,500MHz):δ H 3.31-3.38 (ring, m, 4H); 3.38-3.45 (ring, m, 4H); 3.52-3.62 (ring, m, 8H); 3.76 (CH 2 –COOH,s,4H); 4.14(CH 2 –COOH,s,2H); 4.57(CH 2 – aromatic, s, 2H); 7.71 (aromatic, d, 1H, 3 J HH =8Hz); 7.82 (aromatic, d, 1H, 3 J HH = 8Hz); 7.92 (aromatic, t, 1H, 3 J HH = 8Hz); 13 C{ 1 H} NMR (D with internal dioxane reference 2 O,95℃,125MHz):δ C 49.8 (ring, s); 50.0 (ring, s); 51.5 (ring, s); 51.9 (ring, s); 54.4 (CH 2 –COOH,s); 55.4(CH 2 –COOH,s); 58.6 (CH 2– aromatic, s); 125.0 (aromatic, s); 130.0 (aromatic, s); 141.8 (aromatic, s); 142.2 (aromatic, s); 152.8 (aromatic, s); 170.1 (CO ,s); 172.8(CO,s). HRMS(ESI)m / z:[(M–H) – ](C 20 h 29 BrN 5 o 6 ) Calculated: 514.1307, Measured: 514.1304. E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com