Nitric oxide donor type rivasdil derivative as well as preparation method and application thereof
A compound and configuration technology, applied in the field of medicinal chemistry and pharmacotherapeutics, has good application prospects and the effect of protecting retinal ganglion cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
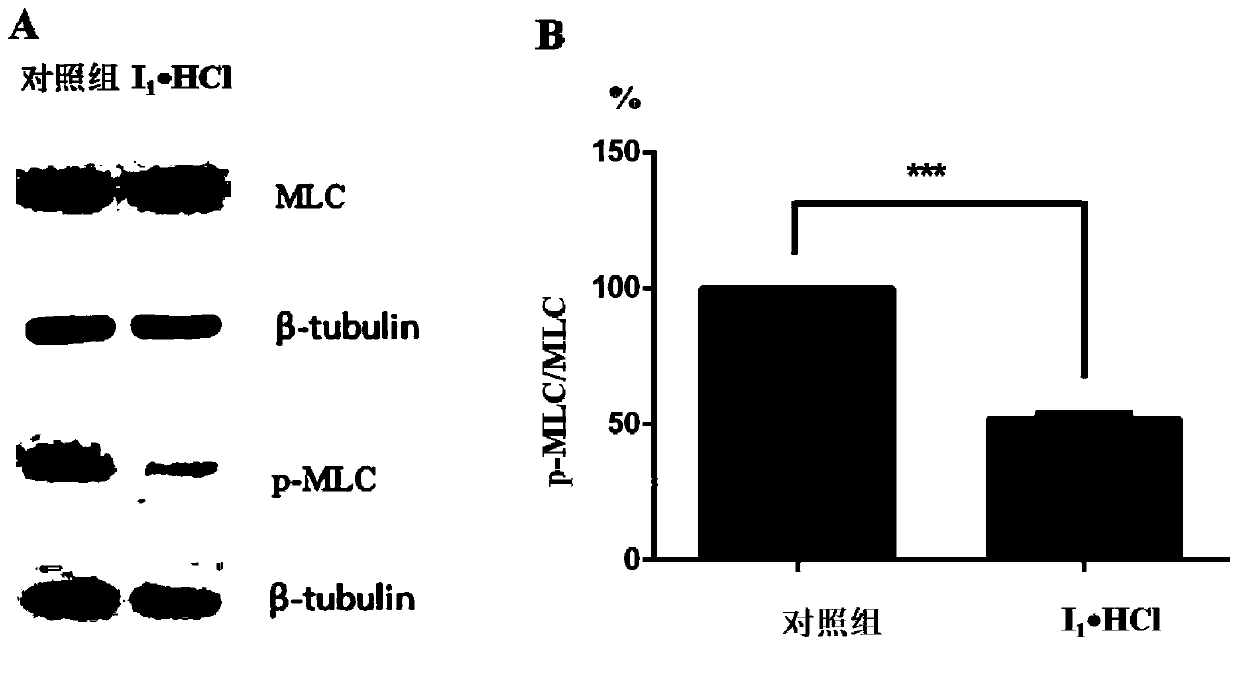
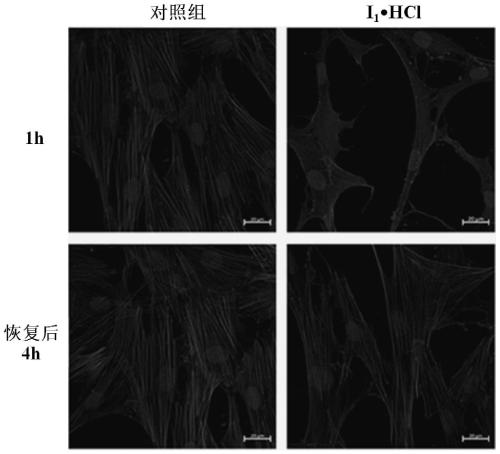
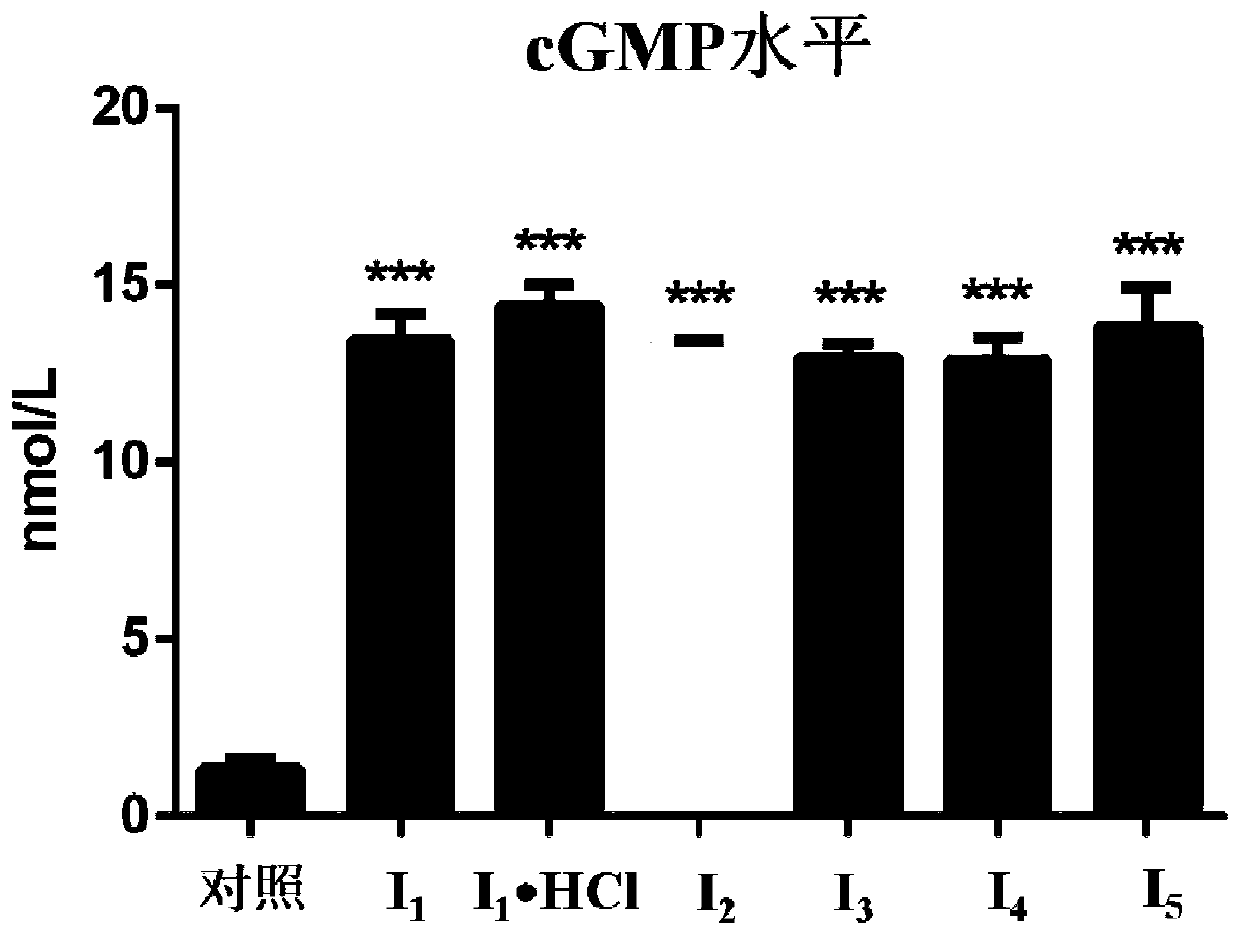
[0072] (S)-4-(Nitrooxy)butan-4-((4-fluoroisoquinolin-5yl)sulfonyl)-3-methyl-1,4-azepane-1-carboxylic acid Esters (I 1 ) and its hydrochloride (I 1 ·HCl)
[0073]
[0074] Dissolve 4-hydroxybutyl nitrate (53mg, 0.39mmol) in 5ml of anhydrous THF, add bis(pentafluorophenyl)carbonate (200mg, 0.51mmol), tetrabutylammonium fluoride (117μl, 0.117mmol ) and stirred at room temperature for 2 h, added TEA (113 μl, 0.78 mmol) to the free compound V ripasudil (154.1 mg, 0.39 mmol) and stirred overnight at room temperature. The reaction solution was diluted by adding an appropriate amount of dichloromethane (50 mL), and washed three times with saturated sodium bicarbonate solution and saturated brine. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain I 1 . 1 H NMR (300MHz, CD 3 OD)δ9.25(s,1H),8.69–8.58(m,1H),8.55(d,J=5.1Hz,1H),8.52–8.44(m,1H),7.94–7.83(m,1H), 4.56(t,J=6.1Hz,2H),4.24(t,J=6.1Hz,2H),4.01–3.85(m,3H),3.44–...
Embodiment 2
[0078] (S)-2-(nitrooxy)ethyl-4-((4-fluoroisoquinolin-5yl)sulfonyl)-3-methyl-1,4-azepane-1-methanol Ester (I 2 )
[0079]
[0080] Dissolve 2-hydroxyethyl nitrate (42mg, 0.39mmol) in 5ml of anhydrous THF, add bis(pentafluorophenyl)carbonate (200mg, 0.51mmol), tetrabutylammonium fluoride (117μl, 0.117mmol ) and stirred at room temperature for 2 h, added TEA (113 μl, 0.78 mmol) to the free compound V ripasudil (154.1 mg, 0.39 mmol) and stirred overnight at room temperature. The reaction solution was diluted by adding an appropriate amount of dichloromethane (50 mL), and washed three times with saturated sodium bicarbonate solution and saturated brine. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain I 2 (50 mg, 32%).
[0081] 1 H NMR (300MHz, CD 3 OD)δ9.25(s,1H),8.73–8.62(m,1H),8.59–8.52(m,1H),8.52–8.44(m,1H),7.88(t,J=7.9Hz,1H), 4.85–4.74(m,2H),4.58–4.34(m,2H),4.30–4.11(m,1H),4.00–3.86(m,2H),3.43–3.31(m,2H)...
Embodiment 3
[0083] (S)-3-(nitrooxy)propyl-4-((4-fluoroisoquinolin-5yl)sulfonyl)-3-methyl-1,4-azepane-1-methanol Ester (I 3 )
[0084]
[0085] Dissolve 3-hydroxypropyl nitrate (47mg, 0.39mmol) in 5ml of anhydrous THF, add bis(pentafluorophenyl)carbonate (200mg, 0.51mmol), tetrabutylammonium fluoride (117μl, 0.117mmol ) and stirred at room temperature for 2 h, added TEA (113 μl, 0.78 mmol) to the free compound V ripasudil (154.1 mg, 0.39 mmol) and stirred overnight at room temperature. The reaction solution was diluted by adding an appropriate amount of dichloromethane (50 mL), and washed three times with saturated sodium bicarbonate solution and saturated brine. The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain I 3 (50 mg, 32%).
[0086] 1 H NMR (300MHz, CD 3 OD)δ9.25(s,1H),8.69–8.60(m,1H),8.60–8.52(m,1H),8.52–8.44(m,1H),7.88(t,J=7.9Hz,1H), 4.74–4.57(m,2H),4.35–4.22(m,2H),4.04–3.88(m,3H),3.45–3.31(m,2H),3.12–2.89(m,2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com