Application of holothurian cerebroside and derivatives thereof in products for improving blood-brain barrier injury
A technology for blood-brain barrier damage and cerebroside, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve problems such as affecting the structure and function of membrane proteins, and achieve good results in reducing blood-brain barrier damage. , the effect of broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
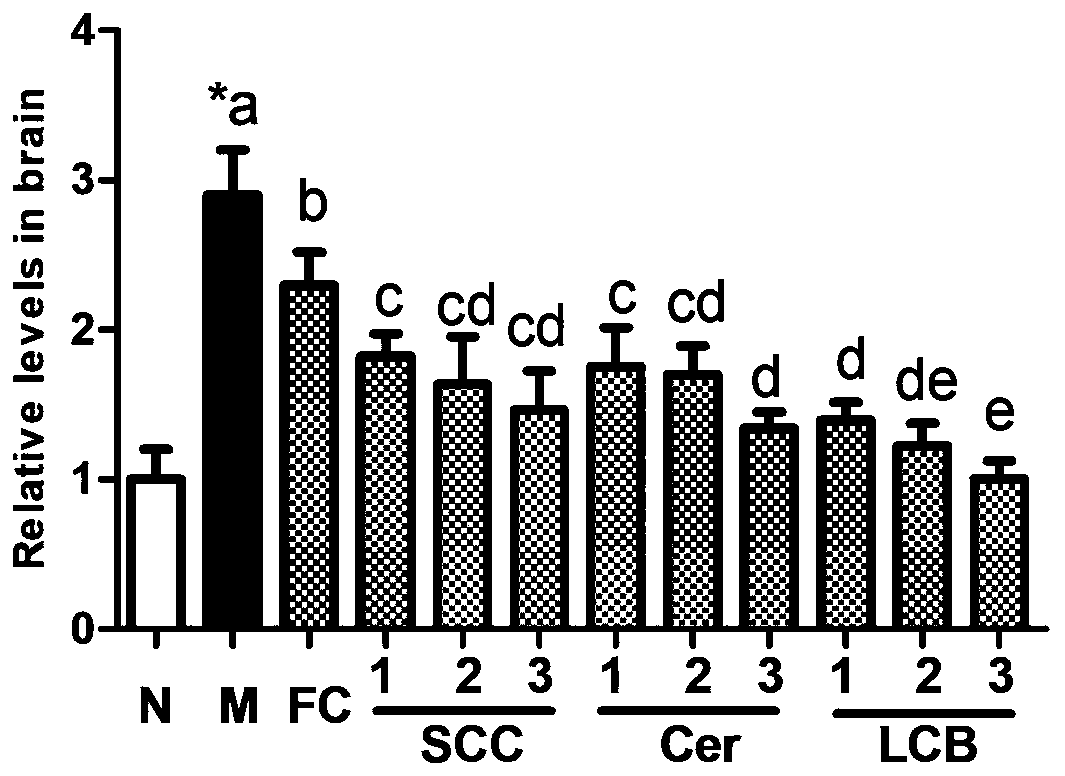
[0027] Example 1: Blood-brain barrier injury induced by ischemia-reperfusion
[0028] 1.1 Experimental method
[0029] 1.1.1 Experimental design
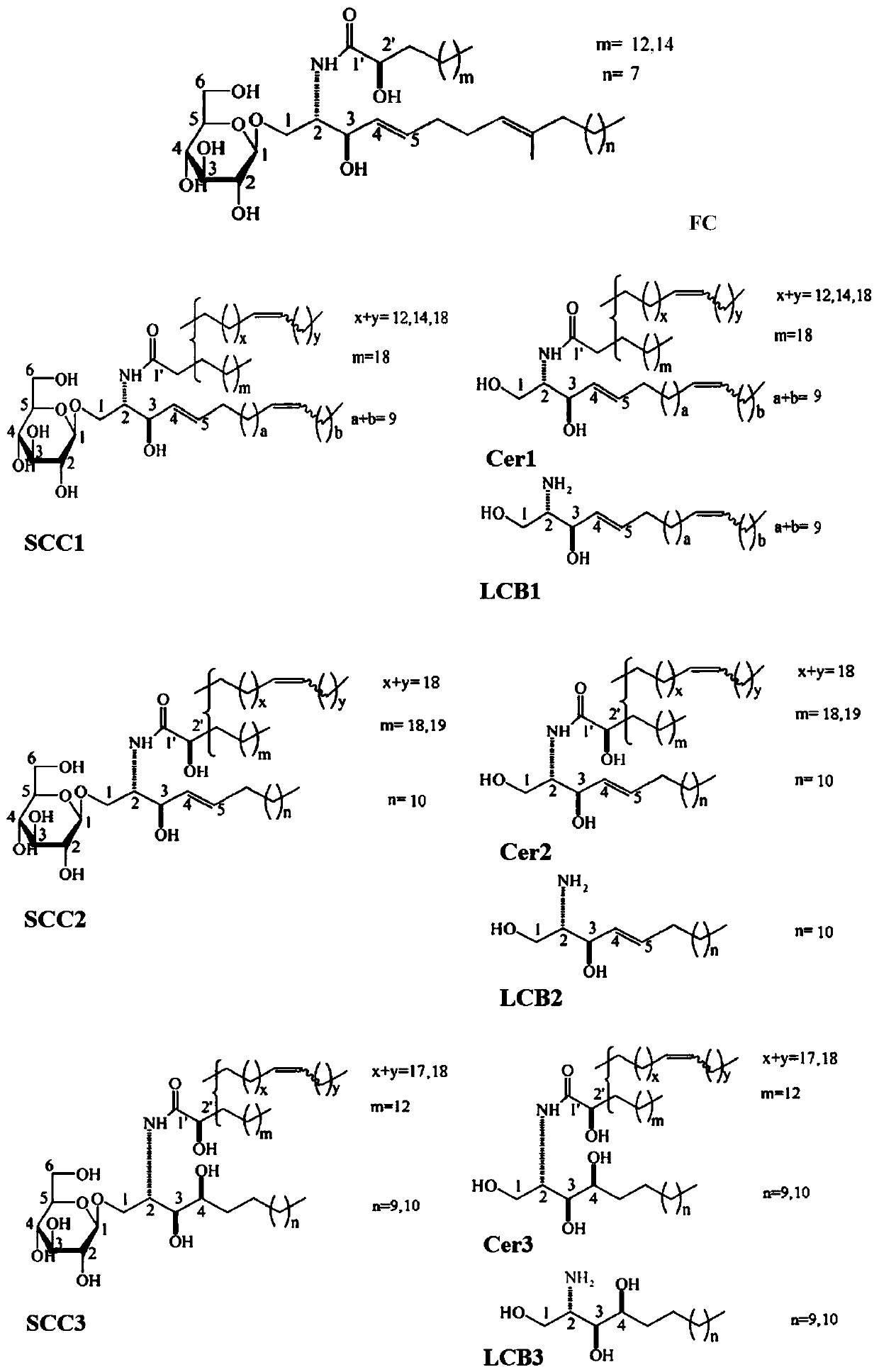
[0030] The experimental animals were male Balb / c mice, weighing 22-23 g, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. The mice were divided into 12 groups: sham operation group (N), model group (M), fungal cerebroside group (FC), three series of sea cucumber cerebrosides (SCC1, SCC2 and SCC3), sea cucumber brain Glycoside derivative ceramide group (Cer1, Cer2 and Cer3) and sea cucumber cerebroside derivative long-chain base group (LCB1, LCB2 and LCB3). Mice in the sham operation group and the model group were fed with modified AIN-93G rodent diet. The mice in the fungal cerebroside group were fed with the feed supplemented with 0.5% fungal cerebroside, and the other groups were fed with the same molar amount of the test substance as in the FC group. Three weeks later, ischemia-reperfusion was per...
Embodiment 2
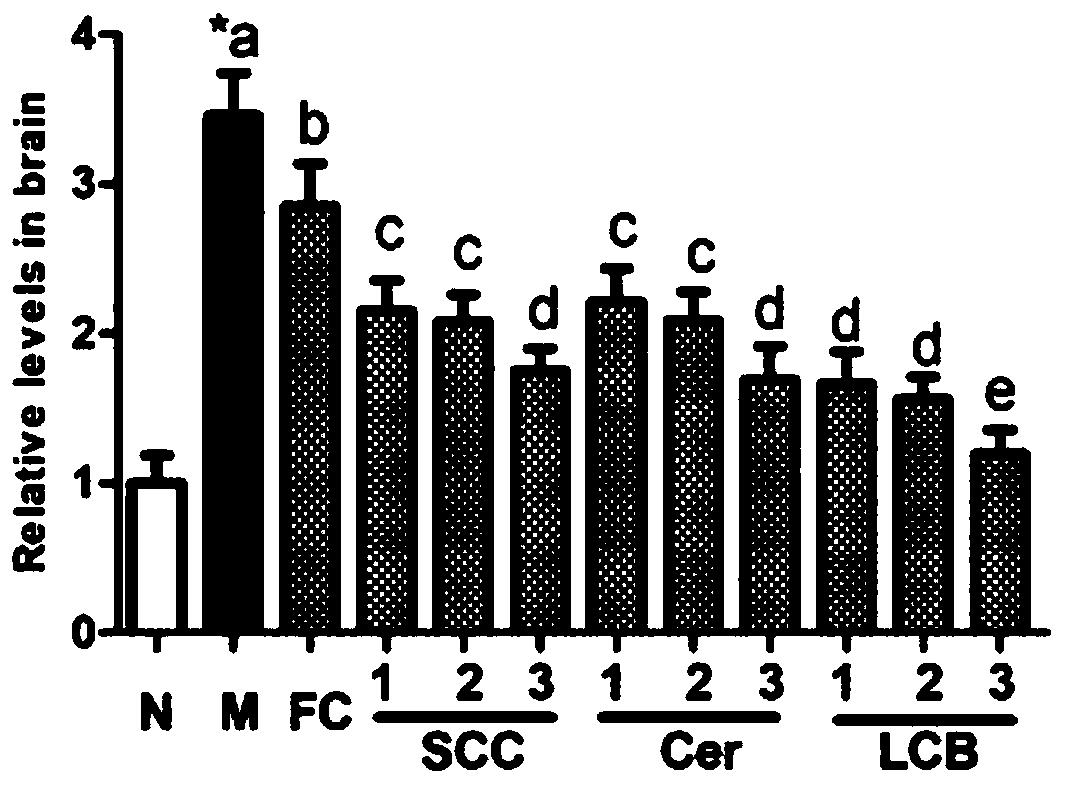
[0034] Example 2: Blood-brain barrier damage induced by lead acetate
[0035] 2.1 Experimental method
[0036] 2.1.1 Experimental design
[0037] The experimental animals were male Balb / c mice, weighing 22-23 g, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. The mice were divided into 12 groups: normal group (N), model group (M), fungal cerebroside group (FC), sea cucumber cerebroside three series groups (SCC1, SCC2 and SCC3), sea cucumber cerebroside Lipid derivative ceramide group (Cer1, Cer2 and Cer3) and sea cucumber cerebroside derivative long-chain base group (LCB1, LCB2 and LCB3). Except the normal group, all the mice were fed with 50 mg / kg lead acetate every day for 9 weeks. Meanwhile, mice were fed a modified AIN-93G rodent diet. After 6 weeks, the mice in the fungal cerebroside group were fed with the feed supplemented with 0.5% fungal cerebroside, and the other groups were fed with the same molar amount of the test substance as the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com