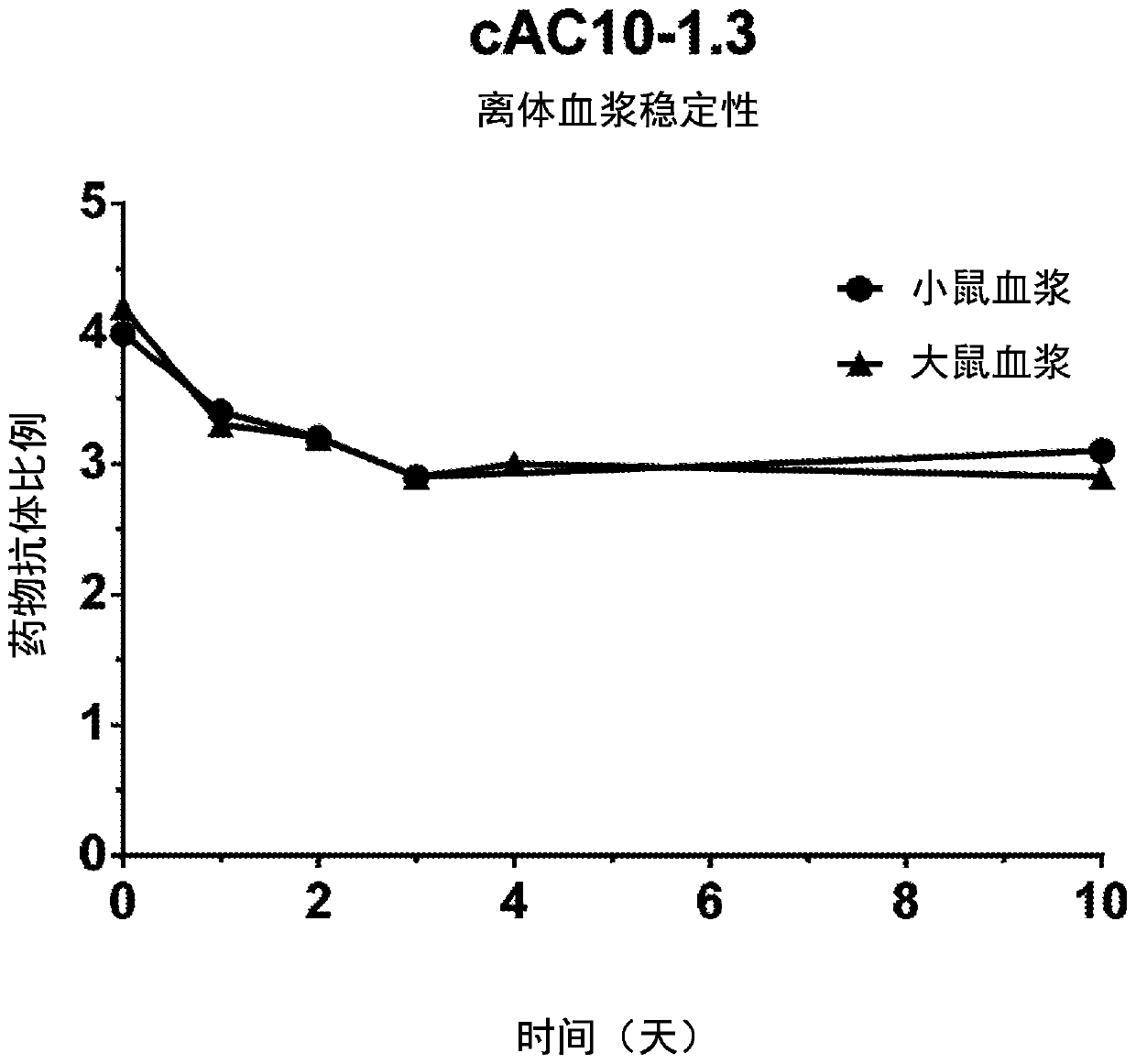
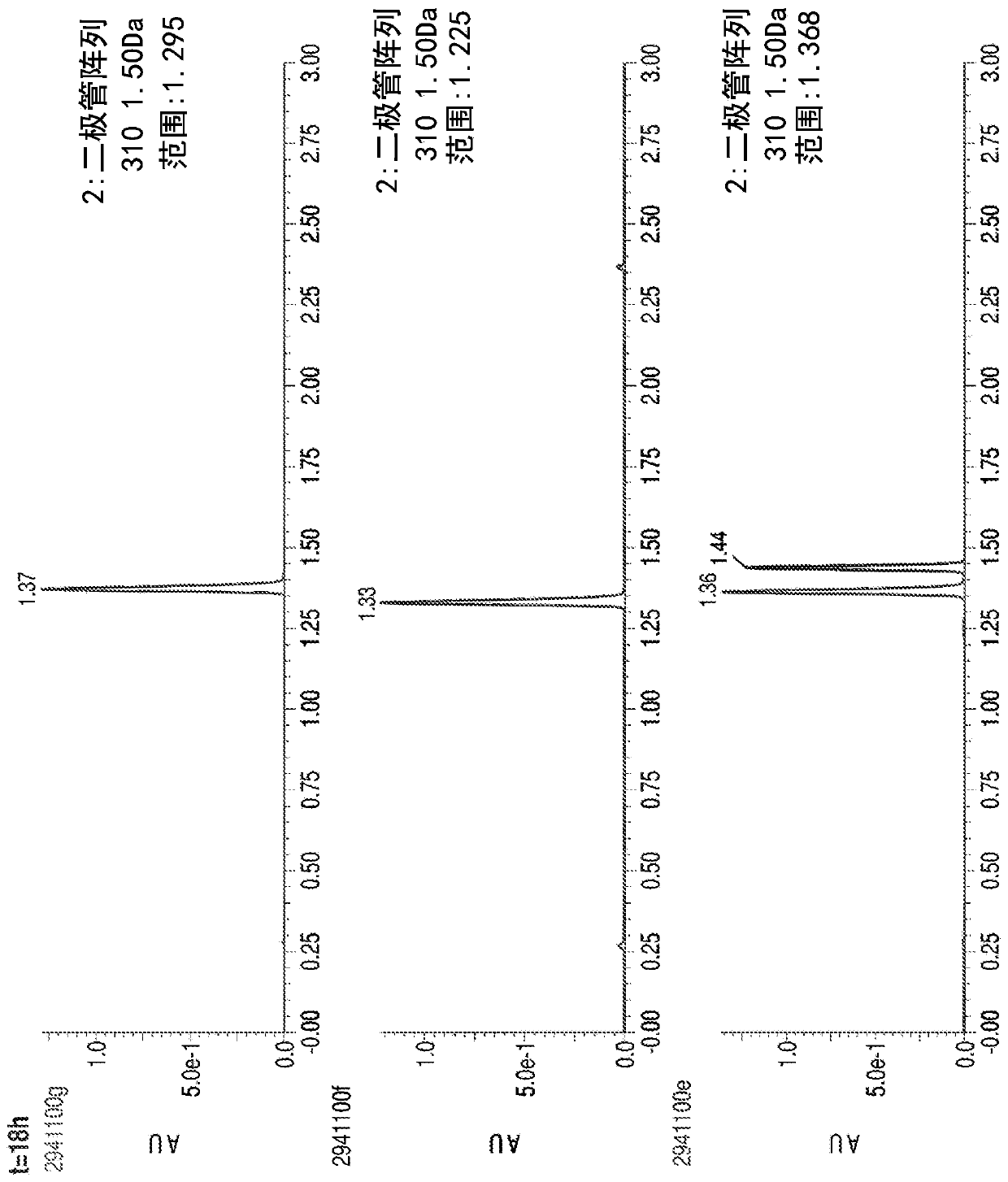
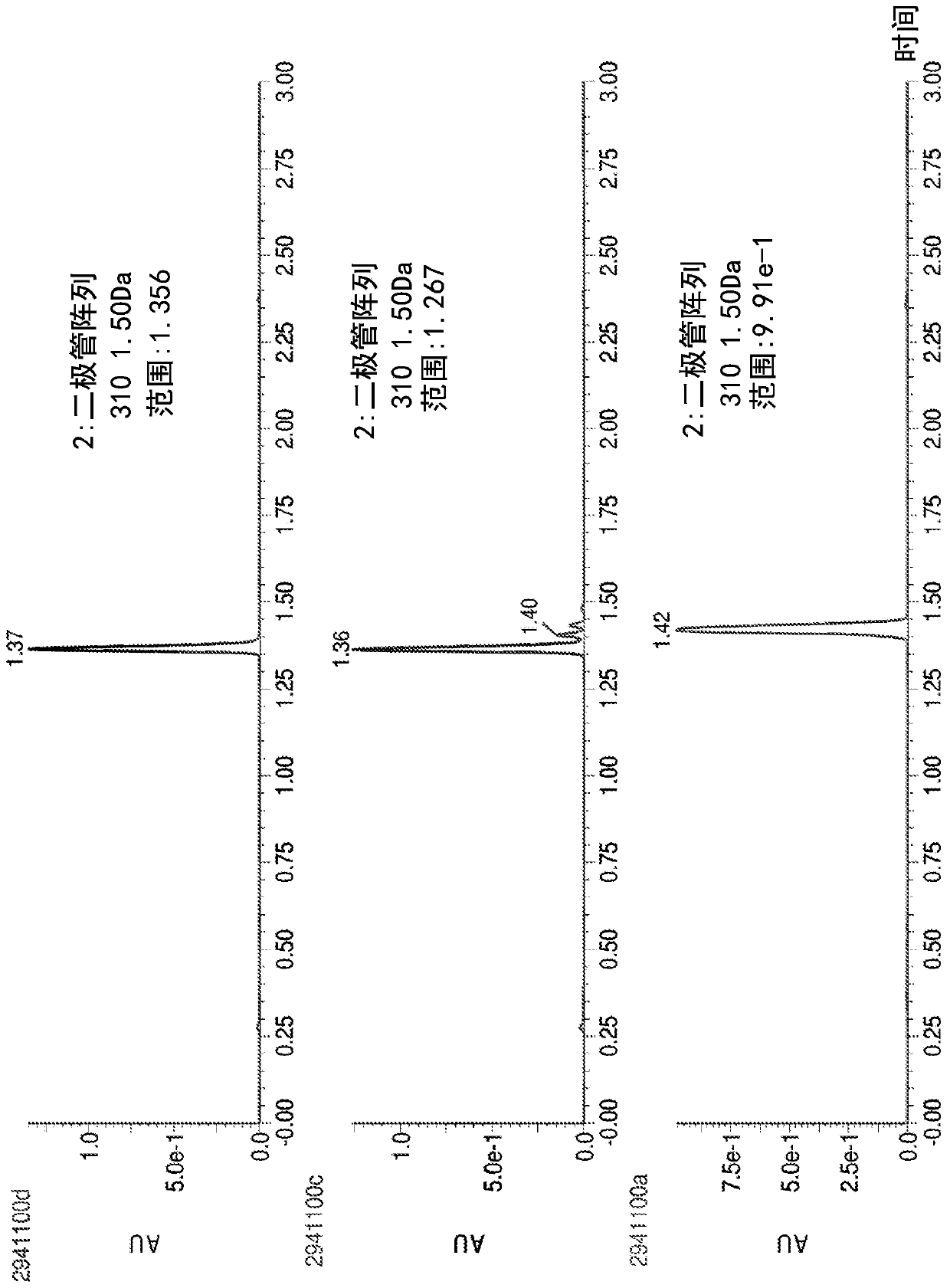
Methylene carbamate linkers for use with targeted-drug conjugates
A technology of methylene carbamate and drug conjugates, which is applied in the field of methylene carbamate linkers used in combination with target-drug conjugates, and can solve the problems of reduced efficacy, increased toxicity, and Insufficient immune specificity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
other Embodiment approach
[0030] Other embodiments are based in part on the discovery that MAC units can be adapted to provide other methylene carbamate units for use with drugs having functional groups other than hydroxyl, including drugs containing sulfhydryl, amide or amine functional groups medicine. Accordingly, exemplary self-disintegrating building units provided herein comprise methylene carbamate units directly attached to heteroatoms from drug functional groups with different leaving group capabilities. In some aspects, the functional groups are hydroxyls (including those of primary, secondary, and tertiary aliphatic and aromatic alcohols), thiols (including alkylthiols and arylthiols), amides (including formamide, sulfonamides and phosphoramides) or amines (including cyclic or alicyclic primary aliphatic amines, secondary aliphatic amines and tertiary aliphatic amines, or primary or secondary aryl amines) to enable attachment to methylene The heteroatom (T*) of the carbamate unit is an oxyg...
Embodiment approach
[0110] Various embodiments of the invention are described below, which are not intended to limit the invention in any way, and are followed by a more detailed discussion of the components that make up the conjugate. It will be appreciated by those skilled in the art that each of the identified conjugates and any of their selected embodiments are intended to encompass the full spectrum of components and linkers.
[0111] Ligand-Drug Conjugates
[0112] In one set of embodiments, provided herein are ligand-drug conjugates (LDCs) and compositions thereof comprising populations of these LDCs (ie, LDC compositions).
[0113] In one aspect, the Ligand-drug conjugate comprises a Ligand unit, a Drug unit, and a Linker unit connecting the Ligand unit to the Drug unit, wherein the Linker unit comprises a self-disintegrating assembly unit, wherein the Ligand unit is passed through The self-disassembling assembly unit is connected to the drug unit. The drug unit is directly linked to th...
Embodiment 1
[0891] Examples 1 and 2 describe the alternative preparation of drug-linker compounds having a drug unit (D) covalently linked to a methylenealkoxycarbamate unit of formula la', wherein the drug unit is derived from Restatin E. The resulting drug-linker compound has the general structure of formula V':
[0892]
[0893] wherein R and R' are hydrogen and the activatable moiety X is -Y(W)-, wherein -Y(W)- has the structure of formula XVId:
[0894]
[0895] where the wavy line to the nitrogen heteroatom of the self-decomposing spacer unit (Y) indicates the covalent attachment to the linker unit (A), and the pound sign (#) indicates the covalent attachment of the benzyl carbon of Y to the MAC unit Linked, where O* is an oxygen heteroatom from the hydroxyl functional group of the free drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com