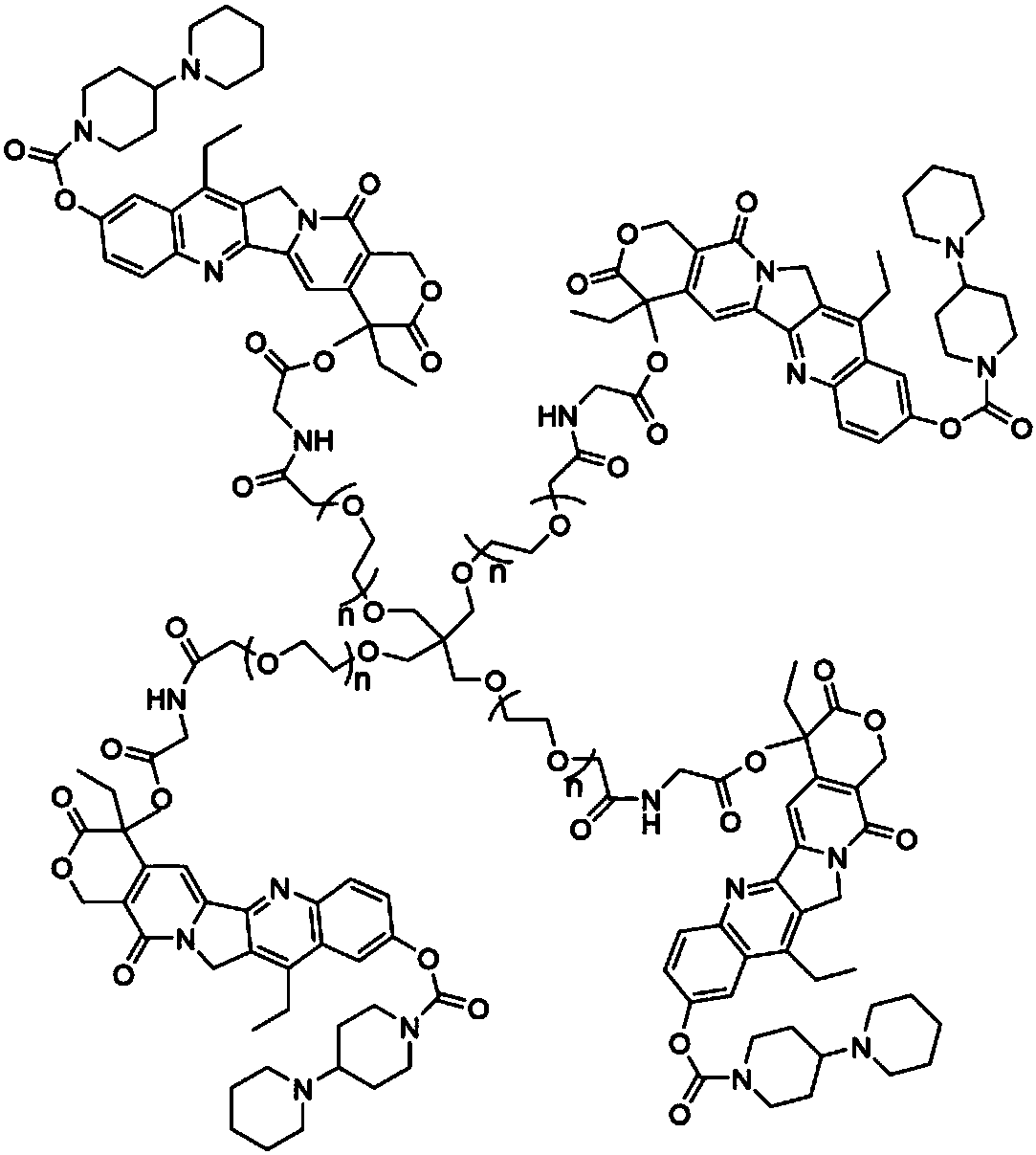
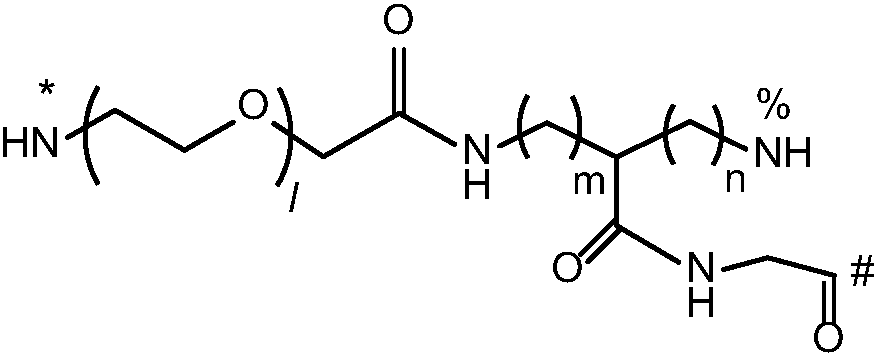
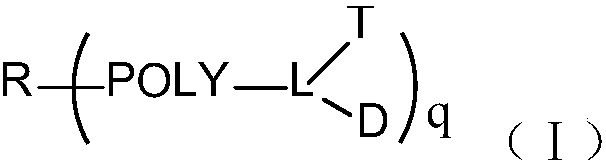Multi-arm targeted anti-cancer conjugate
A technology of drug conjugates and targeting molecules, applied in the field of targeted anti-cancer conjugates, can solve the problems of affecting the performance of normal cells, poor targeting, and high incidence of adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148]
[0149] Preparation of compound 2
[0150] Add 3.50g of compound 1 (1.0eq) and 52.5ml of DMF to a 250mL round bottom flask, heat to 60°C to dissolve, evaporate the DMF under reduced pressure after 5-10min, add 300ml of n-heptane and distill under reduced pressure, repeat three times, spin dry Add 105ml of DCM, 1.08g of Boc-Gly-OH (1.2eq), 63mg of DMAP (0.1eq), add dropwise a solution of 1.59g of DCC (1.5eq) dissolved in 10ml of DCM, and react at 20°C for 4 hours. After the reaction is monitored by TLC, filter and concentrate When the remaining 25% volume was reached, 120ml of IPA was added, 75% of the solvent was evaporated, 150ml of n-heptane was added, stirred at room temperature for 1 hour, filtered, washed twice with n-heptane, and dried to obtain 4.02g of compound 2 as a pale yellow solid.
[0151] Preparation of compound 3
[0152] Add 4.02g of compound 2 and 50ml of DCM to a 100mL three-neck flask, stir and dissolve, then add 11.6ml of TFA dropwise, react at...
Embodiment 2
[0154]
[0155] Preparation of compound 5
[0156] Add 6.9g of compound 4 and 30ml of EA into a 250mL three-neck flask, stir to dissolve and cool down to 0°C, add 40ml of 0.3M HCl / EA, keep the reaction for 2h, monitor the reaction by TLC and concentrate to dryness to obtain compound 5, directly proceed to the following One step reaction.
[0157] Preparation of compound 6
[0158] Dissolve compound 5 (1.0eq) with 50ml of purified water, add 3.96g of sodium bicarbonate (2.0eq), dissolve 5.30g of Fmoc-OSU (1.0eq) with 50ml of DME, add it to the reaction flask of compound 5, and add 25ml of THF , stirred at room temperature for 2 hours, after the reaction was monitored by TLC, evaporated the organic solvent, extracted impurities with EA, adjusted the pH of the aqueous phase to 3-4 with dilute hydrochloric acid, extracted twice with EA, combined the organic phases, washed once with water, and washed with saturated saline It was dried over anhydrous sodium sulfate and concentr...
Embodiment 3
[0164] Preparation of Targeting Molecule cRGD (Compound 11) Linked with Protecting Group
[0165]
[0166] Preparation of compound 9
[0167] Using 2Cl-Trt Resin, Fmoc protection method, HOBT / DIC as the coupling reagent, DMF as the reaction solvent, and ninhydrin detection as the reaction monitoring, the following protected amino acids are connected to the resin in sequence: Fmoc-Gly-OH, Fmoc-Arg (Pbf)-OH, Fmoc-Glu(OBzl)-OH, Fmoc-D-Phe-OH, Fmoc-Asp(OtBu)OH, remove Fmoc, wash with DMF, DCM, wash with methanol and dry, then add lysis reagent: Acetic acid / TFE / DCM=1 / 2 / 7, reacted for 2 hours, precipitated with ice MTBE, washed, and dried to obtain compound 9 as an off-white solid.
[0168] Preparation of compound 10
[0169] Add 14.0g of compound 9 (1.0eq) to a 2L three-necked flask, add 1L of DMF, cool to 0°C, add 9.2g of sodium bicarbonate (8.0eq), add 15.1g of DPPA (4.0eq) after dissolving, and keep warm overnight. After the TLC reaction was completed, it was poured into 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com