Antibody drug conjugate of CLDN 18.2-resistant antibody and preparation method and application of antibody drug conjugate
A technology of conjugates and drugs, which is applied in the field of biomedicine and can solve problems such as lack of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241] Example 1: Construction of high-expression cell lines of claudin 18.1, 18.2 (CLDN18.1, CLDN18.2)
[0242] The human CLDN18.1, human CLDN18.2, mouse CLDN18.1, and mouse CLDN18.2 high-expression cell lines used in the present invention are completed through the company's stable cell line construction platform. Specific steps are as follows:
[0243] On the first day of the experiment, 293T cells (Cat#GNHu17, a cell bank of the Type Culture Collection Committee of the Chinese Academy of Sciences) were seeded in two 6cm culture dishes, and the number of cells in each culture dish reached 7.5×10 5 indivual. On the second day, the plasmids (pGag-pol, pVSV-G, pBabe, etc. BioVector, Plasmid Vector Strain Cell Gene Collection Center) and the plasmid pBabe-CLDN18.2 or pBabe for cloning the human or mouse CLDN18.2 or CLDN18.1 gene will be wrapped. - Add OPTI-MEM (Thermofisher Scientific Cat#31985070) to 4 μg each of CLDN18.1 to make the final volume 200 μl, prepare 200 μl OPTI-M...
Embodiment 2
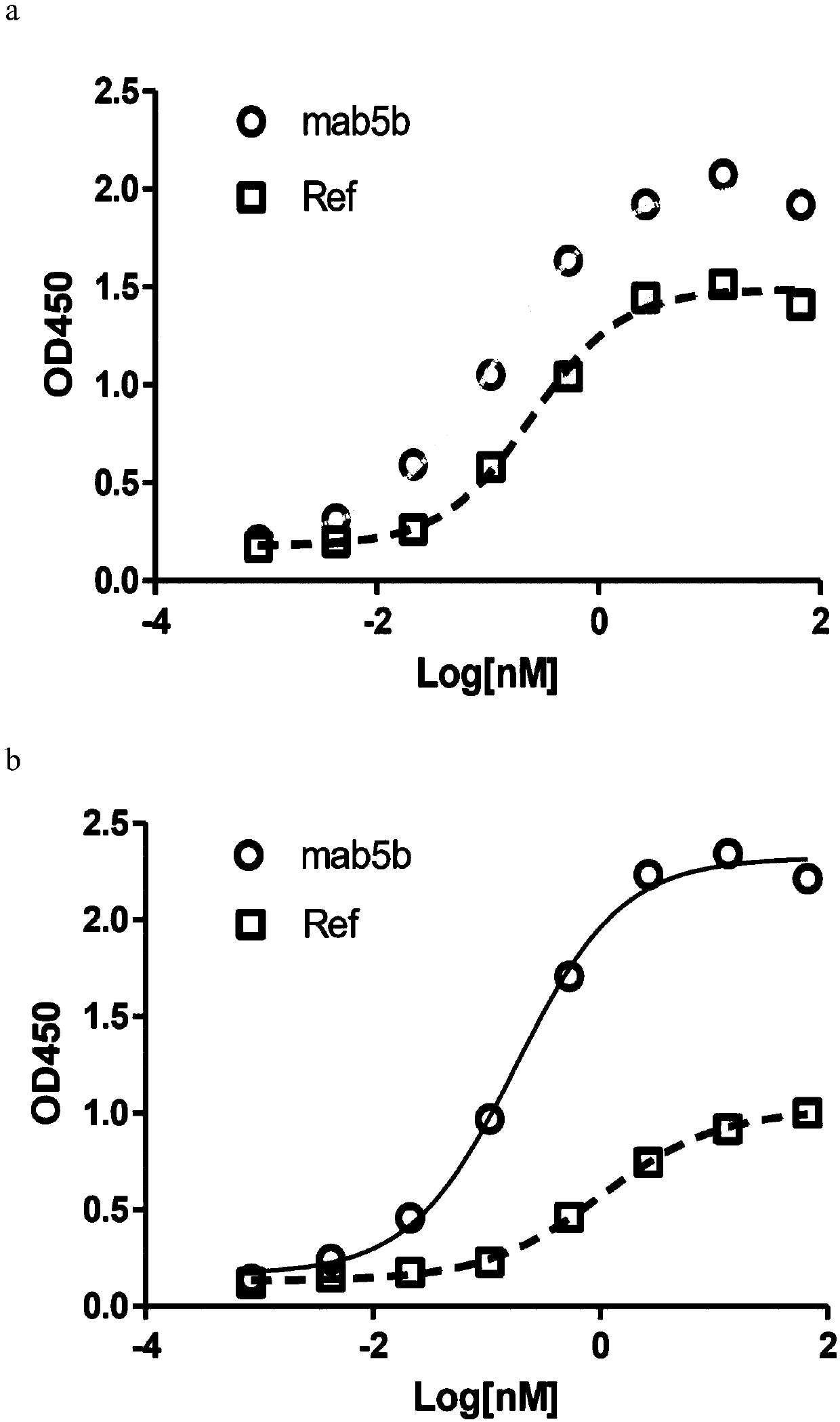
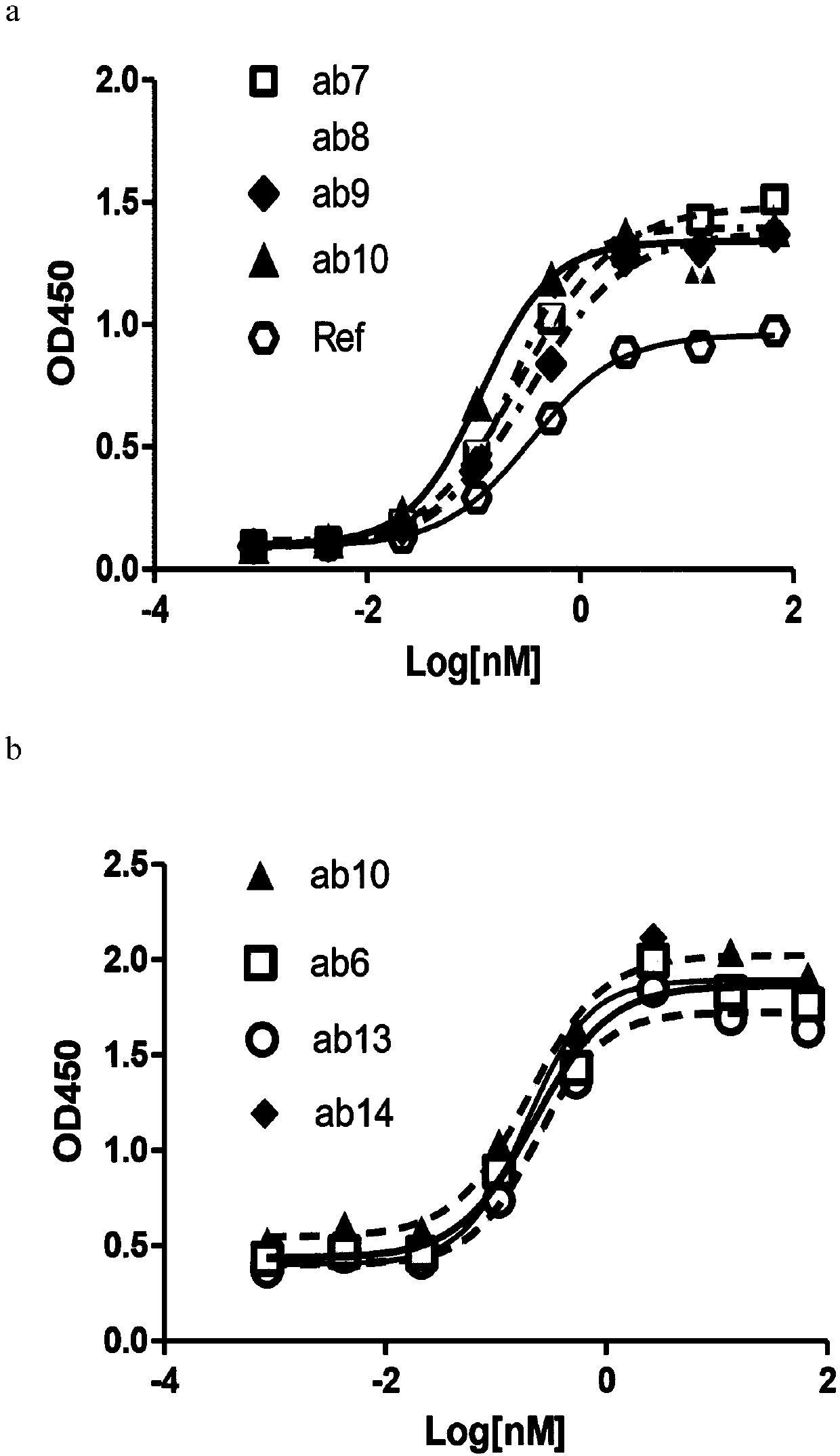
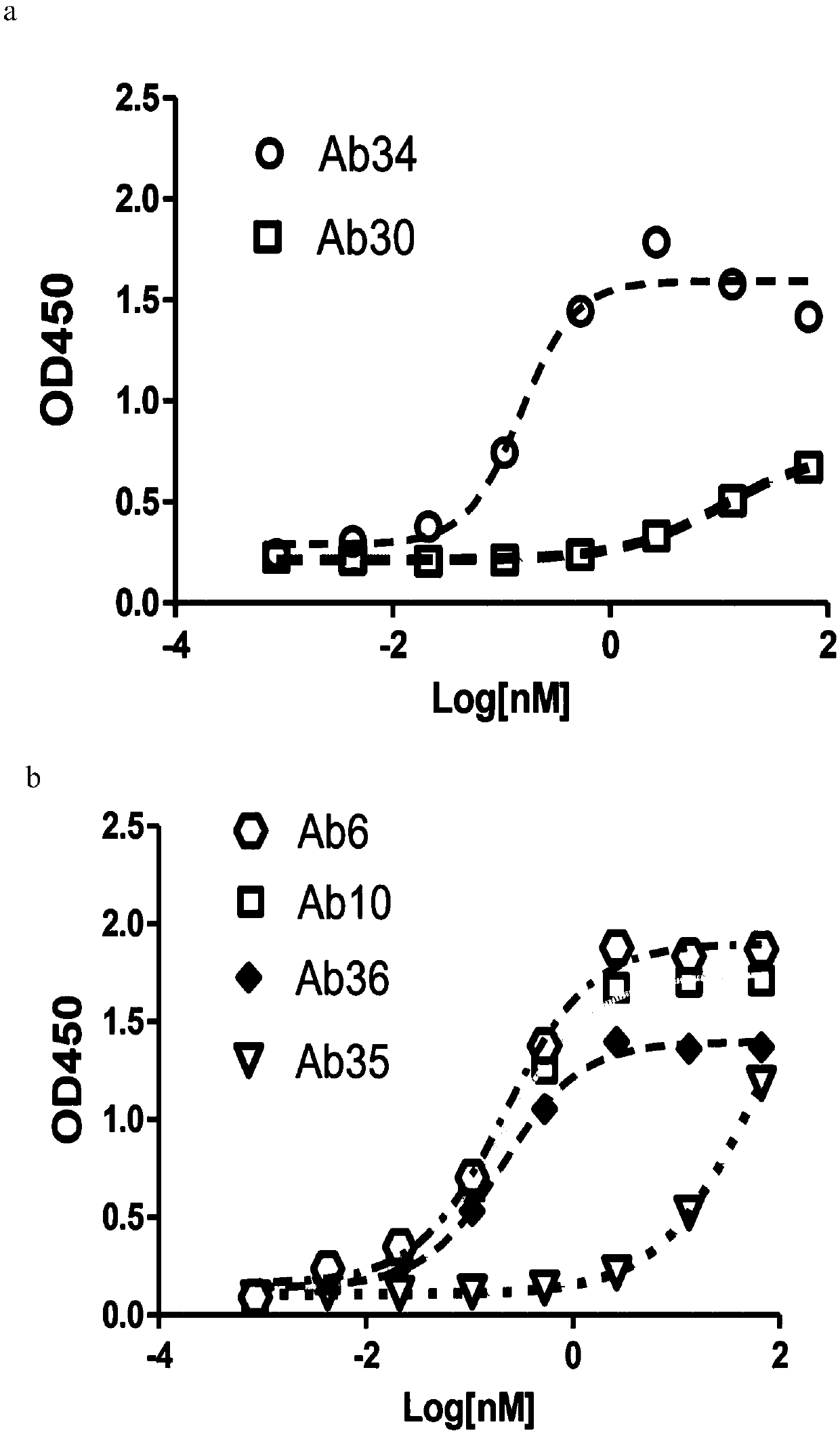
[0257] Example 2: Anti-CLDN18.2 antibody and CLDN18.2+ and CLDN18.1+ cell line binding (ELISA) experiment
[0258] After expanding the monoclonal cell line with high expression of human CLDN18.1, human CLDN18.2, mouse CLDN18.1 or mouse CLDN18.2 obtained in Example 1, press 5×10 4 96-well plate was spread per well, and the supernatant was removed after overnight incubation in a 37°C incubator, and fixed with 100 μl / well of immunostaining fixative (Shanghai Beyotime Biotechnology Co., Ltd. Cat#P0098) for half an hour at room temperature. After washing once with PBS (Yuanpei Biology, Cat#B320), block with 5% milk at 37°C for 2 hours, and wash 3 times with PBST. Add the sample to be tested (human or mouse antibody, Jackson Immuno Research). Incubate at 37°C for 1 hour, then wash 3 times with PBST. Add Anti-human or mouse HRP 1:2500 50μl / well and incubate at 37°C for 1 hour, then wash 3 times with PBST, develop color with TMB (Surmodic Cat#TTMB-1000-01), add 50μl / well 1M H 2 SO ...
Embodiment 3
[0259] Example 3: Cloning, expression and purification of recombinant proteins and antibodies
[0260] The cloning, expression and purification of the recombinant protein / antibody used in the present invention are carried out according to molecular cloning methods well known to those skilled in the art.
[0261] Specifically, the expression vector used in the present invention was purchased from Changsha Youbao Biotechnology Co., Ltd., and then introduced EcoRI enzyme cleavage site (GAATTC) by Shanghai Jianxin Biomedical Technology Co., Ltd. (Jinxin Biotechnology) to facilitate double enzyme digestion Or homologous recombination method to clone foreign genes. Gene synthesis is completed by companies such as Sangon Bioengineering (Shanghai) Co., Ltd. (Sangon Biotechnology). 293 cells and CHO-K were purchased from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences.
[0262] The recombinant protein and antibody in the present invention are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com