Method for determining relative correction factor of methanol in methoxymethanol without standard sample and application
A relative correction factor, methoxymethanol technology, applied in the field of gas chromatography detection, can solve the problems of methoxymethanol instability, difficulty in purchasing standard samples, and inability to determine the correction factor, etc., and achieve the effect of accurate results and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
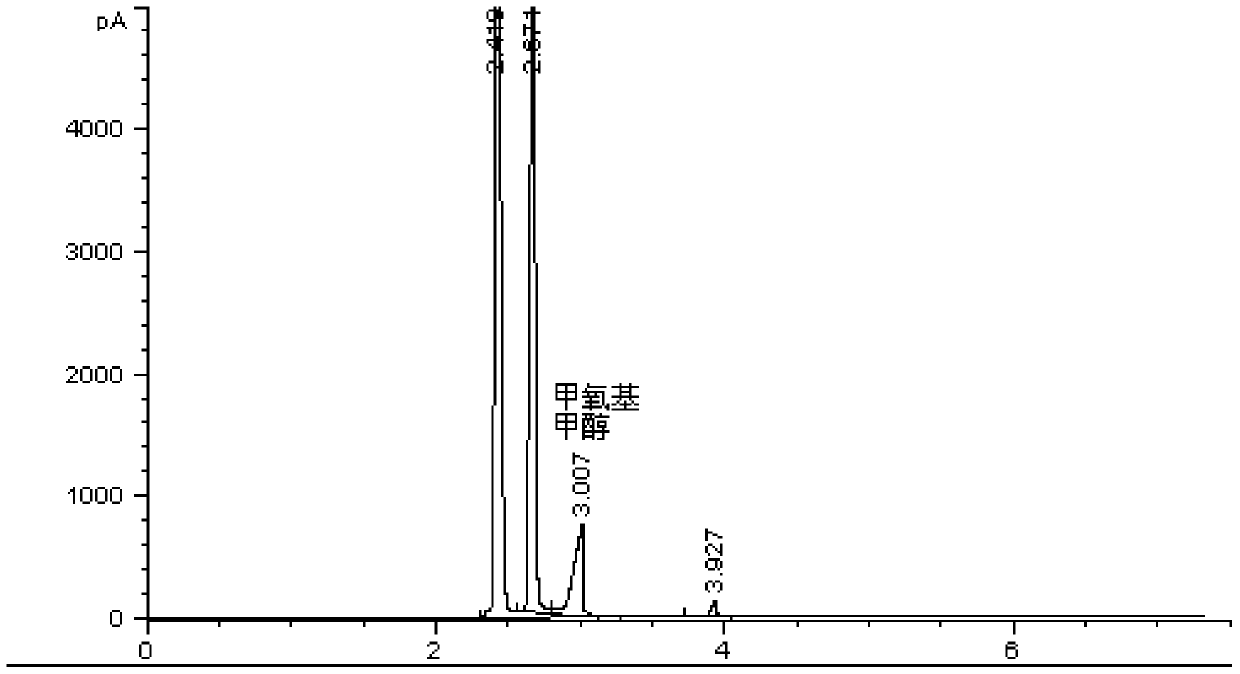
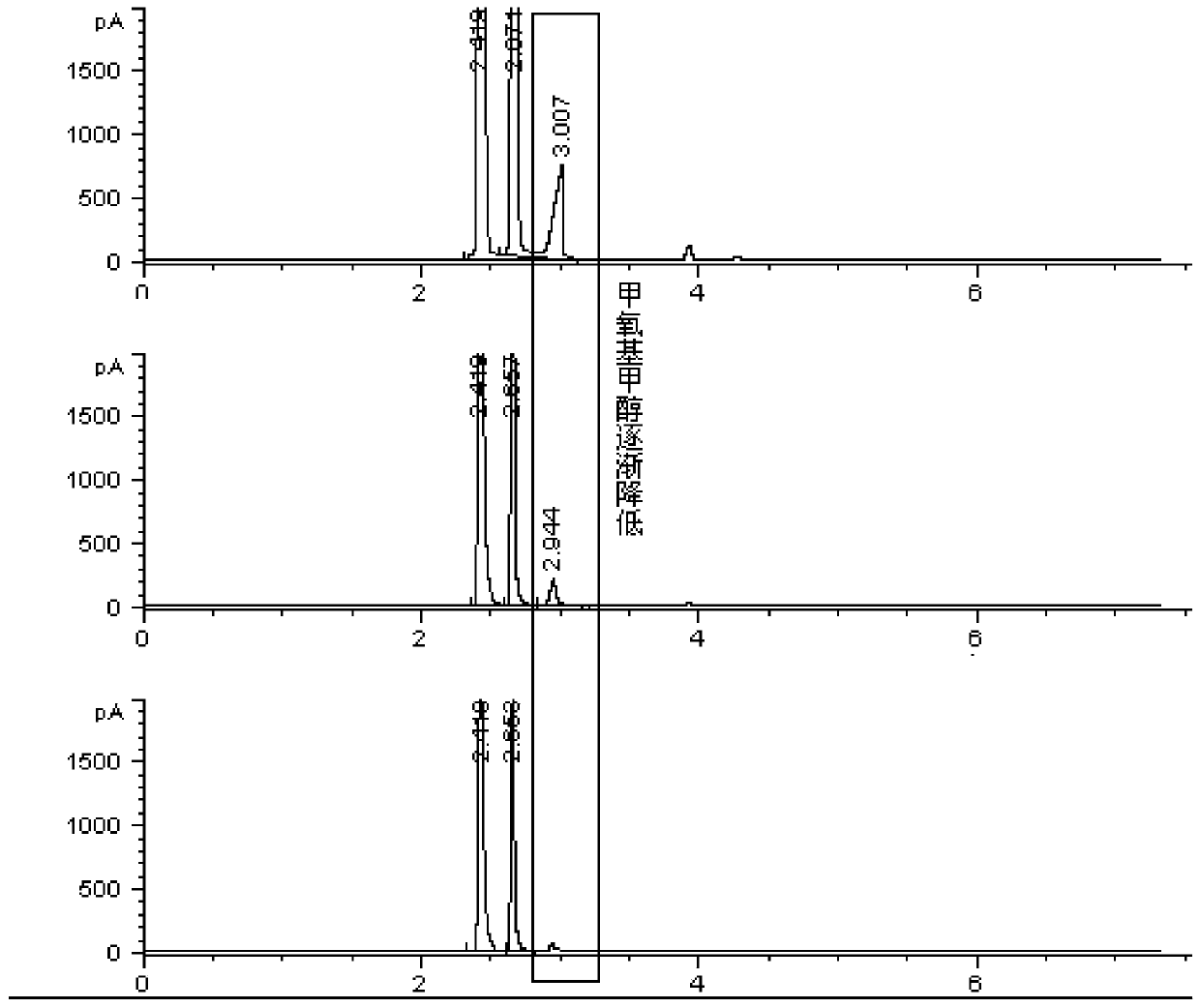
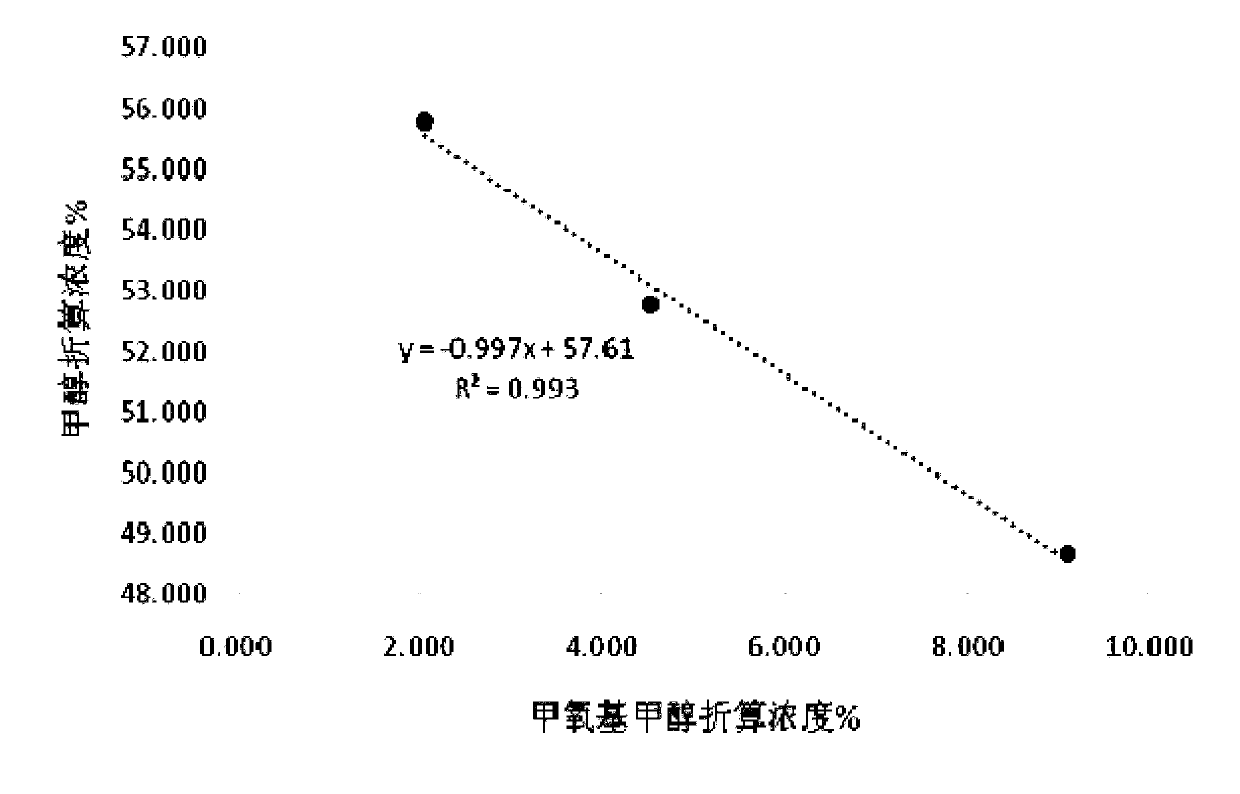
[0032] Take methanol-stabilized concentrated formaldehyde (containing water, methanol, and methoxymethanol), add isopropanol, mix well, and inject samples on a weakly polar chromatographic column HP-5 and an FID detector to determine methanol, isopropanol, and isopropanol. The area-normalized content of propanol and methoxymethanol; add sodium sulfite to the solution, mix well and inject the sample again, and record the area-normalized content of methanol, isopropanol and methoxymethanol; add sodium sulfite again, mix well Afterwards, the sample was injected again, and the area-normalized content of methanol, isopropanol and methoxymethanol was recorded. The experimental results and calculation results are shown in Table 1: because the solution contains traces of other impurities, the sum of the contents of the three is not 100%. The contents of the three were normalized; due to the difference in the response factors of methanol in methoxymethanol and free methanol, the normali...
example 2
[0037]Take methanol-stabilized concentrated formaldehyde (containing water, methanol, and methoxymethanol), add ethanol, mix well, inject samples on a highly polar chromatographic column INNOWAX, and FID detector, and record methanol, ethanol, and methoxyl The area-normalized content of methanol; add sodium sulfite to the solution, mix well and inject again, and record the area-normally content of methanol, ethanol, and methoxymethanol; add sodium sulfite again, mix well and inject again, record methanol, The area-normalized content of ethanol and methoxymethanol, the experimental results and calculation results are shown in Table 2: Since the solution contains a small amount of other impurities, the sum of the contents of the three is not 100%, and the contents of the three are normalized; Due to the difference in the response factors of methanol in methoxymethanol and free methanol, there is a difference in the normalized content of ethanol. After adding sodium sulfite, the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com