The production method of methyl o-formate benzylsulfonamide
A technology of methyl benzyl sulfonamide and methyl benzyl sulfonyl chloride, which is applied in the field of pesticide intermediates, can solve the problems of high cost and low yield, and achieve the effect of reducing production and increasing product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
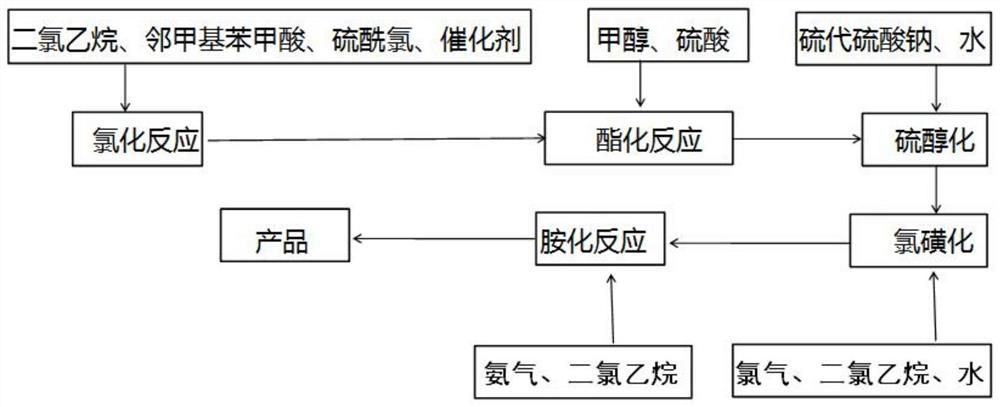
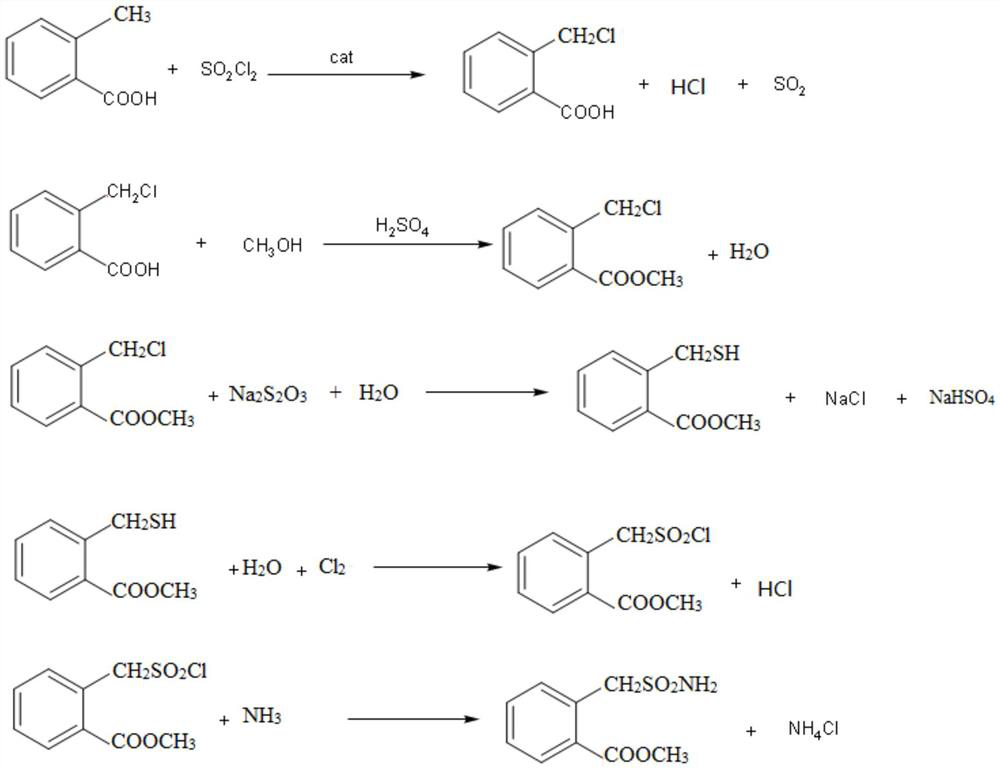
[0045] (1) the synthesis of o-chloromethyl benzoic acid: in reactor, add solvent dichloroethane 500kg, then add o-toluic acid 300kg, and catalyzer azobisisobutyronitrile 4kg, steam is warming up to 60 ℃ of refluxes, Add chlorinating agent sulfuryl chloride 350kg dropwise, carry out reflux reaction, react for 4 hours, and synthesize o-chloromethyl benzoic acid under catalysis; After the reaction, the solvent is recovered by distillation, then add clear water and stir to cool down for crystallization, and filter and dry to obtain o-chloromethyl benzoic acid. 368kg of benzoic acid crystals, content 97%, yield 98%;
[0046] (2) synthesis of methyl o-chloromethyl benzoate: 368kg of o-chloromethyl benzoic acid synthesized by step (1) is added in the esterification reactor, then 400kg of methanol is added, and 150kg of vitriol oil is added under stirring to be a catalyzer, steam Heating to 80°C to carry out esterification reaction, the reaction was completed for 6 hours, the unreacte...
Embodiment 2
[0052] (1) synthesis of o-chloromethyl benzoic acid: in reactor, add solvent dichloroethane 500kg, then add o-toluic acid 300kg, and catalyzer azobisisobutyronitrile 4kg, steam is warming up to 70 ℃ of backflow, Add chlorinating agent sulfuryl chloride 360kg dropwise, carry out reflux reaction, react for 4 hours, and synthesize o-chloromethyl benzoic acid under catalysis; After the reaction, the solvent is recovered by distillation, then clear water is added, stirring is lowered, and temperature crystallization is performed, and o-chloromethyl benzoic acid is obtained by filtration and drying. 365kg of benzoic acid crystals, content 98%, yield 97%;
[0053] (2) synthesis of methyl o-chloromethyl benzoate: 365kg of o-chloromethyl benzoic acid synthesized by step (1) is added in the esterification reactor, then 400kg of methanol is added, and 150kg of vitriol oil is added under stirring to be a catalyst, steam Heating to 60° C. to carry out esterification reaction, the reaction ...
Embodiment 3
[0059] (1) the synthesis of o-chloromethyl benzoic acid: in reactor, add solvent dichloroethane 500kg, then add o-toluic acid 300kg, and catalyzer azobisisobutyronitrile 4kg, steam is warming up to 60 ℃ of refluxes, Add 365 kg of chlorinating agent sulfonyl chloride dropwise, carry out reflux reaction, react for 4 hours, and synthesize o-chloromethyl benzoic acid under catalysis; after the reaction, the solvent is recovered by distillation, then add clear water and stir to lower temperature for crystallization, and filter and dry to obtain o-chloromethyl benzoic acid. 368kg of benzoic acid crystals, content 97%, yield 98%;
[0060] (2) synthesis of methyl o-chloromethyl benzoate: 368kg of o-chloromethyl benzoic acid synthesized by step (1) is added in the esterification reactor, then 400kg of methanol is added, and 150kg of vitriol oil is added under stirring to be a catalyzer, steam Heating to 100°C to carry out esterification reaction, the reaction was over for 6 hours, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com