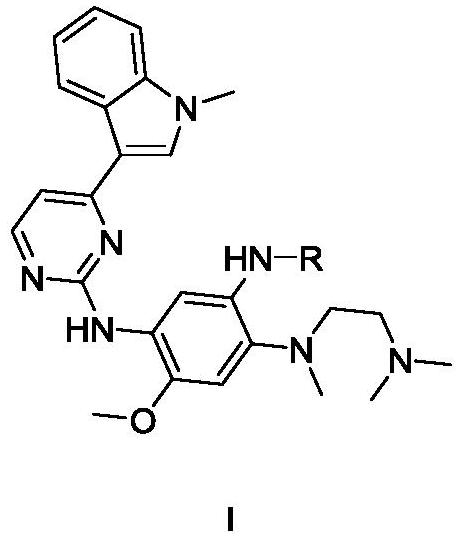
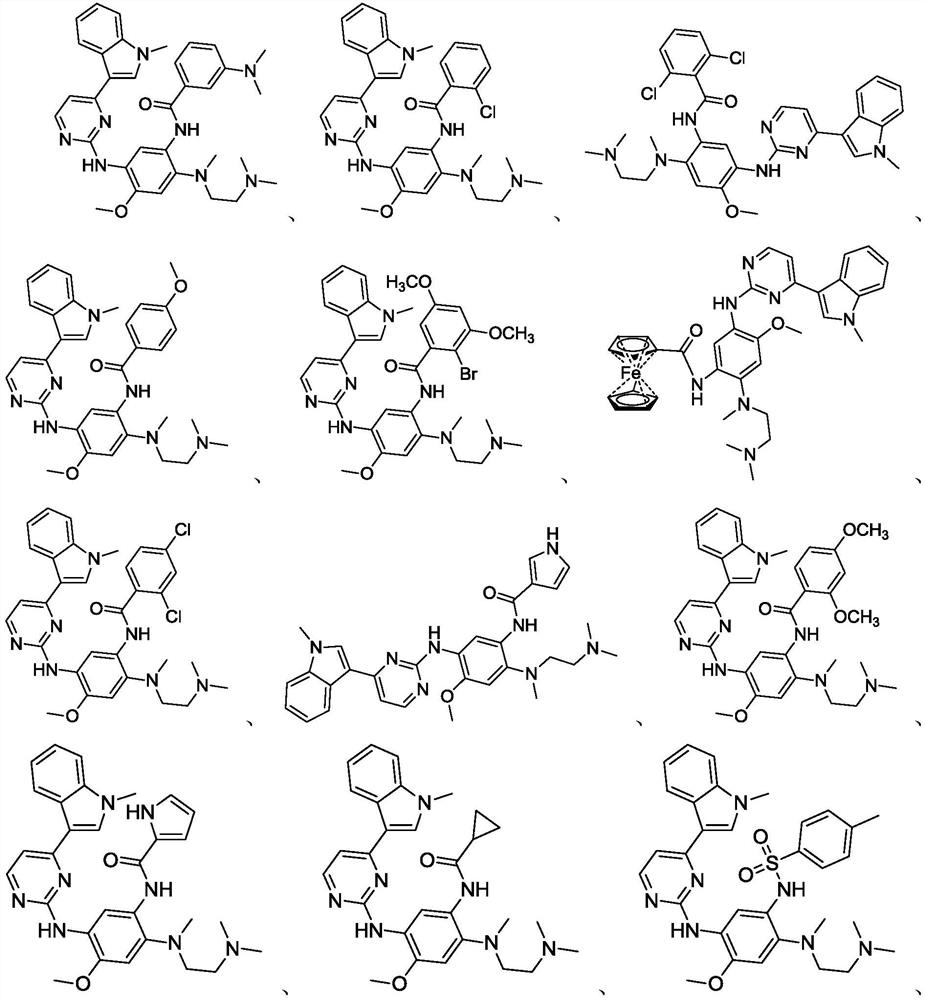
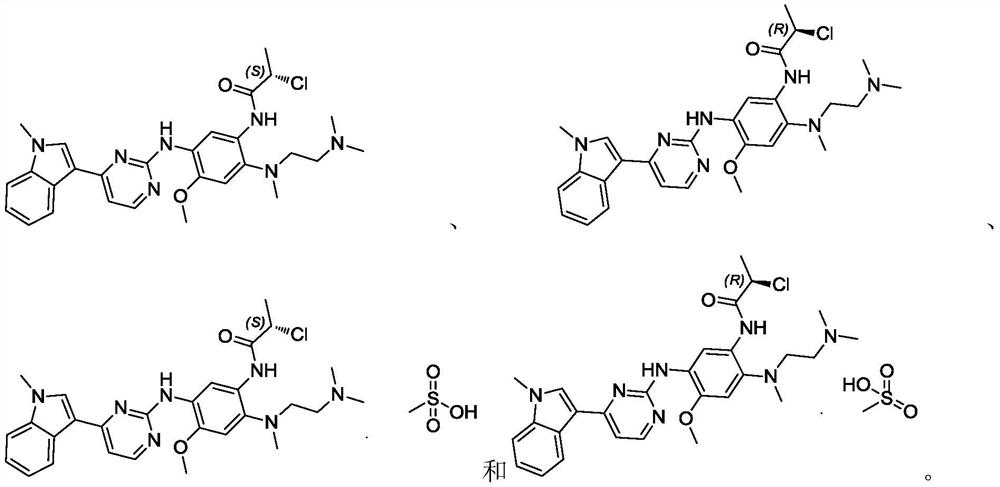
Application of methoxy-substituted phenylamide aminopyrimidine derivative
A phenyl and methyl technology, applied in the application field of methoxy substituted phenyl amide aminopyrimidine derivatives, can solve the problems of EGFR kinase toxicity, drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1 N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3 Preparation of -yl)-2-pyrimidinyl]amino]phenyl]-2-(3-N,N-dimethylamino)benzamide
[0092]
[0093] The compound N1-(2-(dimethylamino)ethyl)-5-methoxy-N1-methyl-N4-(4-(1-methyl-1H-indol-3-yl)pyrimidine- 2-yl)benzene-1,2,4-triamine (1.0g, 2.24mmol, 1eq), 3-(N,N-dimethylamino)benzoic acid (445mg, 2.69mmol, 1.2eq) and HATU (1.28 g, 3.37mmol, 1.5eq) was dissolved in dichloromethane (20ml), DIPEA (1.11ml, 6.73mmol, 3.0eq) was added, and after stirring at room temperature for 4 hours, the reaction solution was washed three times with water, dried and concentrated, and the crude product was subjected to column chromatography ( dichloromethane / methanol) to give the product (1.33g).
[0094] 1 H NMR (400MHz, DMSO-d 6 ):δ=10.37(s,1H),9.30(s,1H),8.79(s,1H),8.36(d,J=5.2Hz,1H),8.25(d,J=8.0Hz,1H),7.91 (s,1H),7.53(d,J=8.0Hz,1H),7.36(t,J=8.0Hz,1H),7.29-7.19(m,4H),7.19-7.12(m,1H),7.11( s,1...
Embodiment 2
[0095] Example 2 N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3 Preparation of -yl)-2-pyrimidinyl]amino]phenyl]-2-(2-chloro)benzamide
[0096]
[0097] Reference Example 1 prepares N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-ind Indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-(2-chloro)benzamide (1.31g)
[0098] 1 H NMR (400MHz, DMSO-d 6 ):δ=10.91(s,1H),9.22(s,1H),8.70(s,1H),8.35(d,J=5.2Hz,1H),8.25(d,J=8.0Hz,1H),7.96 (s,1H),7.63(dd,J=7.2,2.4Hz,1H),7.59(dd,J=7.6,1.6Hz,1H),7.55-7.45(m,3H),7.28-7.21(m,2H ),7.19-7.12(m,1H),7.10(s,1H),3.87(s,3H),3.86(s,3H),2.89(s,2H),2.74(s,3H),2.16(s, 2H), 1.76ppm (s, 6H); ES-API (m / z): calculated value C 32 h 34 ClN 7 o 2 [M+H]+, 584.2; theoretical value, 584.2.
Embodiment 3
[0099] Example 3 N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-indole-3 -yl)-2-pyrimidinyl]amino]phenyl]-2-(2,6-difluoro)benzamide
[0100]
[0101] With reference to Example 1, N-[2-[[2-(dimethylamino)ethyl]methylamino]-4-methoxy-5-[[4-(1-methyl-1H-ind Indol-3-yl)-2-pyrimidinyl]amino]phenyl]-2-(2,6-difluoro)benzamide (1.29 g).
[0102] 1 H NMR (400MHz, DMSO-d 6 ):δ=11.12(s,1H),9.17(s,1H),8.64(1H),8.35(d,J=5.2Hz,1H),8.24(d,J=8.0Hz,1H),7.97(s ,1H),7.65-7.55(m,1H),7.52(d,J=8.0Hz,1H),7.33-7.21(m,4H),7.20-7.12(m,1H),7.10(s,1H), 3.87(s,3H),3.85(s,3H),2.89(t,J=5.6Hz,2H),2.74,(s,3H),2.19(t,J=5.6Hz,2H),1.81ppm(s ,6H); ES-API(m / z): calculated value C 32 h 33 f 2 N 7 o 2 [M+H]+, 586.3; theoretical value, 586.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com