Pharmaceutical formulations for treating endometriosis, uterine fibroids, polycystic ovary syndrome or adenomyosis
A drug and composition technology, applied in the direction of medical preparations containing active ingredients, drug combinations, drug delivery, etc., can solve problems such as adenomyosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0268] Example 1: Compound A monosodium salt forms a gel
[0269] To estimate the solubility of Compound A in water, various amounts of Compound A sodium salt were added to a fixed volume of 1.5 mL and equilibrated at 37°C; the concentration of Compound A in the solution was analyzed.
[0270] Table 2 lists the raw data and observations of the experiments, and figure 2 Concentrations are shown as a function of the amount of Compound A solid added. figure 2 The dashed line in is the theoretical concentration based on the weight of solids added and the volume of water. Such as figure 2 As shown in , the concentration of Compound A is in agreement with the simple calculation, reaching 100 mg solid / 1.5 mL. The deviation of the concentration from the theoretical line is caused by the volume expansion when a large amount of solute dissolves. Apart from that, although the concentration deviated from the theoretical line, the solution was still clear and no significant gelling ...
example 2
[0274] Example 2: In vitro release in the absence of anti-gelling agents
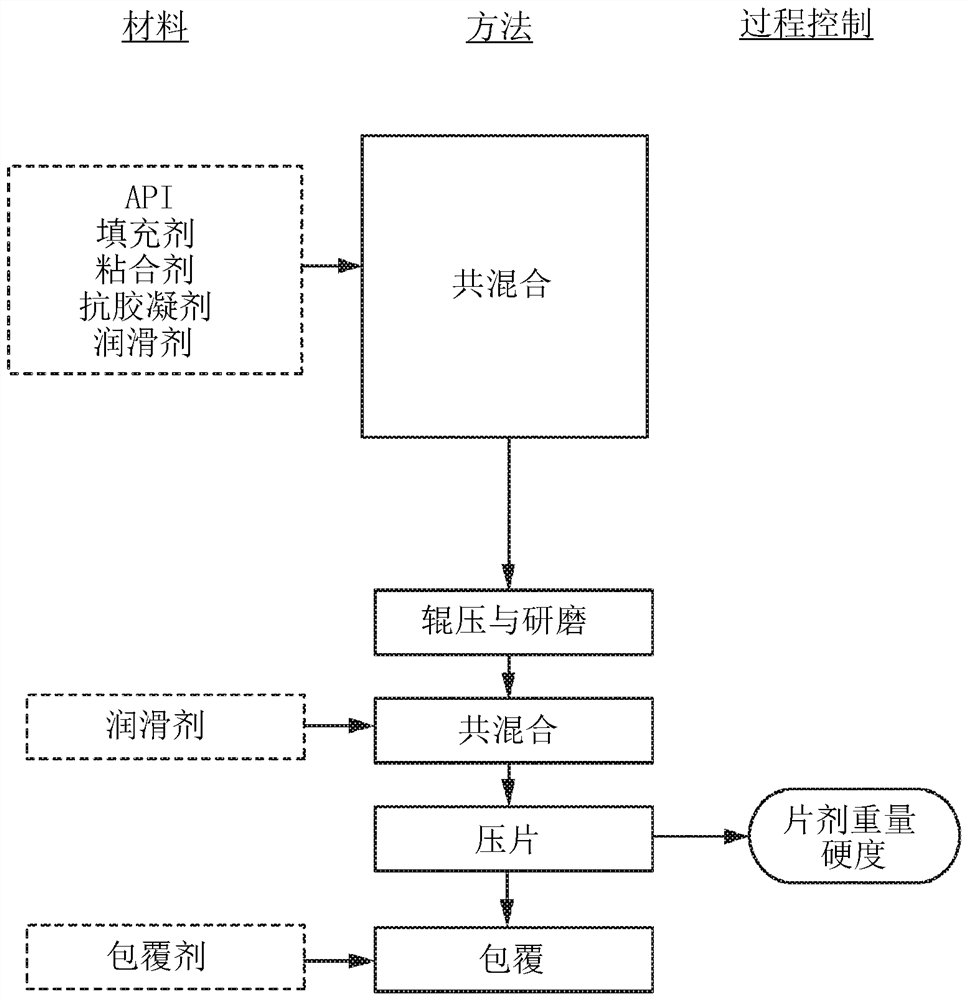
[0275] Immediate release formulations were prepared without anti-gelling agents. All ingredients except magnesium stearate were blended in a high shear granulator and granulated with purified deionized water. The granules were pan dried at 40°C and passed through a #20 US Standard Sieve and lubricated with magnesium stearate. Compound A mentioned in the table below is Compound A sodium salt.
[0276] Composition of formulations without antigelling agents
[0277] Element Quantity (mg / tablet) Compound A, sodium salt 207.3 Mannitol 304.0 pregelatinized starch 59.1 Povidone K 29 / 32 18.4 Magnesium stearate 11.2
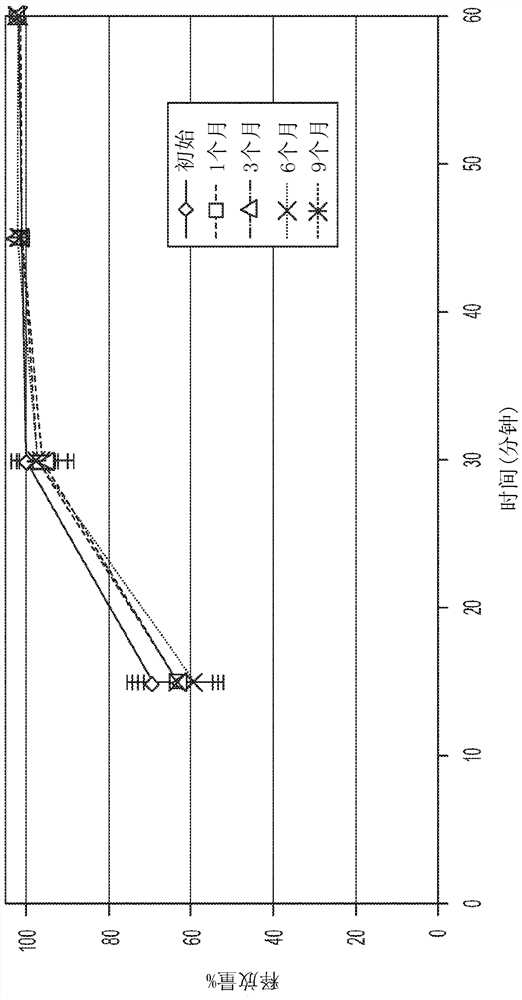
[0278] Table 3 shows the dissolution profile of the uncoated tablets in pH 1.2 media.
[0279] Table 3: (RC2i; 200 mg; Lot No. 170123A-01 (GLIMS #39746))
[0280] time (minutes) Mean % (Standard Deviation) 15 15(0.5) 30 31(0.5...
example 3
[0281] Example 3: Formulations with anti-gelling agents
[0282] Table 4 provides additional non-limiting examples of components of the disclosed formulations and the weight percent (w / w) of these components in the final coated tablet. Compound A mentioned in the table below is Compound A sodium salt, and the corresponding amounts (mg / tablet) and weight percentages are provided in terms of the salt form.
[0283] Table 4. Composition of exemplary formulations.
[0284]
[0285] a Percentages given are based on the weight of the coated tablet. Total percentages may not be 100% due to rounding.
[0286] Table 4. Composition of Exemplary Formulations (continued)
[0287]
[0288] a Percentages given are based on the weight of the coated tablet. Total percentages may not be 100% due to rounding.
[0289] Table 4. Composition of Exemplary Formulations (continued)
[0290]
[0291] a Percentages given are based on the weight of the coated tablet. Total percentages...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com