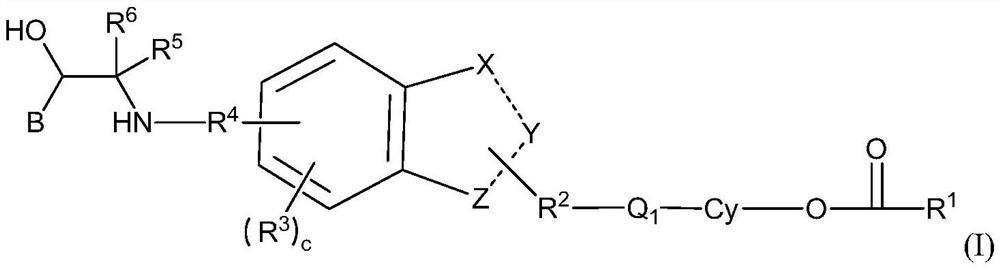
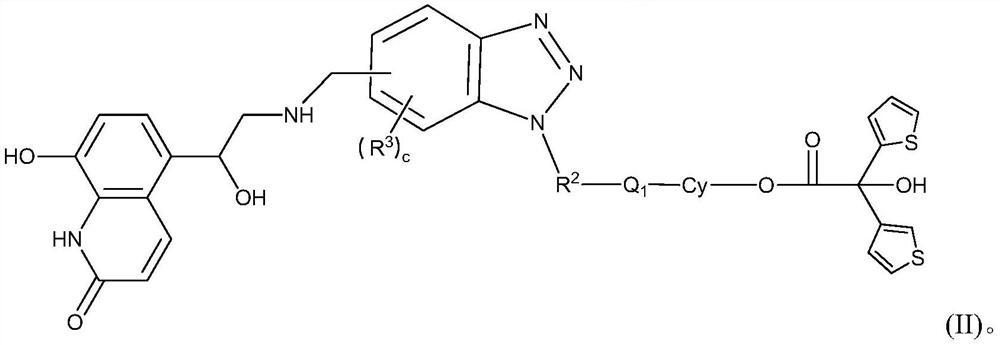
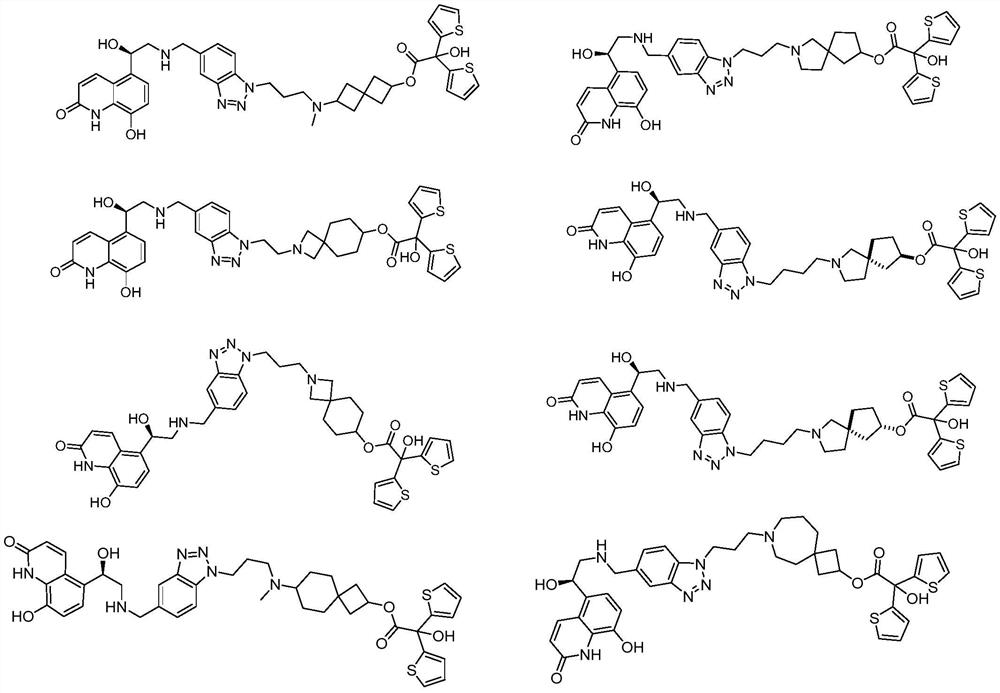
Spiro-containing derivative with beta2 receptor agonist and M receptor antagonist activity and medical application of the spiro-containing derivative
A stereoisomer and pharmaceutical technology, applied in the field of spirocycle-containing derivatives with β2 receptor agonistic and M receptor antagonistic activities and their application in medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] [6-[3-[5-[[[(2R)-2-Hydroxy-2-(8-Hydroxy-2-oxo-1H-quinolin-5-yl)ethyl]amino]methyl]benzene Triazol-1-yl]propylamino]spiro[3.3]heptane-2-yl]-2-hydroxyl-2,2-bis(2-thienyl)acetate ditrifluoroacetate (compound 1 )
[0230] [6-[3-[5-[[[(2R)-2-hydroxy-2-(8-hydroxy-2-oxo-1H-quinolin-5-yl)ethyl]amino]methyl]benzotriazol-1-yl ]propylamino]spiro[3.3]heptan-2-yl]-2-hydroxy-2,2-bis(2-thienyl)acetate ditrifluoroacetate
[0231]
[0232] The first step: [6-[tert-butoxycarbonyl(methyl)amino]spiro[3.3]heptane-2-yl]-2-hydroxyl-2,2-bis(2-thienyl)acetate ( 1b)
[0233] [6-[tert-butoxycarbonyl(methyl)amino]spiro[3.3]heptan-2-yl]-2-hydroxy-2,2-bis(2-thienyl)acetate
[0234]
[0235] Dissolve tert-butyl N-(2-hydroxyspiro[3.3]heptan-6-yl)-N-methylcarbamate (1a) (0.5 g, 2.07 mmol) in toluene (20 mL), add Sodium hydride (0.311g, 7.77mmol) was stirred for 30min, methyl 2-hydroxy-2,2-bis(2-thienyl)acetate (0.66g, 2.59mmol) was added, and the temperature was raised to 115°C for 10 hours....
Embodiment 2
[0254] [2-[2-[5-[[[(2R)-2-Hydroxy-2-(8-Hydroxy-2-oxo-1H-quinolin-5-yl)ethyl]amino]methyl]benzene Triazol-1-yl]ethyl]-2-azaspiro[3.5]non-7-yl]-2-hydroxy-2,2-bis(2-thienyl)acetate ditrifluoroacetic acid Salt (compound 2)
[0255] [2-[2-[5-[[[(2R)-2-hydroxy-2-(8-hydroxy-2-oxo-1H-quinolin-5-yl)ethyl]amino]methyl]benzotriazol-1-yl ]ethyl]-2-azaspiro[3.5]nonan-7-yl]2-hydroxy-2,2-bis(2-thienyl)acetate ditrifluoroacetate
[0256]
[0257] The first step: tert-butyl 7-[2-hydroxy-2,2-bis(2-thienyl)acetyl]oxy-2-azaspiro[3,5]nonane-2-carboxylate (2b)
[0258] Tert-butyl 7-[2-hydroxy-2,2-bis(2-thienyl)acetyl]oxy-2-azaspiro[3.5]nonane-2-carboxylate
[0259]
[0260] Dissolve tert-butyl 7-hydroxy-2-azaspiro[3.5]nonane-2-carboxylate (1.5g, 6.26mmol) in toluene (60mL), add sodium hydride (0.180g, 7.52mmol), and stir for 30 After 10 minutes, methyl 2-hydroxy-2,2-bis(2-thienyl)acetate (1.75 g, 6.89 mmol) was added, and the temperature was raised to 115° C. for 10 hours. The reaction w...
Embodiment 3
[0279] [2-[3-[5-[[[(2R)-2-Hydroxy-2-(8-Hydroxy-2-oxo-1H-quinolin-5-yl)ethyl]amino]methyl]benzene Triazol-1-yl]propyl]-2-azaspiro[3.5]non-7-yl]-2-hydroxy-2,2-bis(2-thienyl)acetate ditrifluoroacetic acid Salt (compound 3)
[0280] [2-[3-[5-[[[(2R)-2-hydroxy-2-(8-hydroxy-2-oxo-1H-quinolin-5-yl)ethyl]amino]methyl]benzotriazol-1-yl ]propyl]-2-azaspiro[3.5]nonan-7-yl]-2-hydroxy-2,2-bis(2-thienyl)acetate ditrifluoroacetate
[0281]
[0282] The first step: [2-[3-(5-formylbenzotriazol-1-yl)propyl]-2-azaspiro[3.5]non-7-yl]-2-hydroxyl-2,2 -Bis(2-thienyl)acetate (3a)
[0283] [2-[3-(5-formylbenzotriazol-1-yl)propyl]-2-azaspiro[3.5]nonan-7-yl]-2-hydroxy-2,2-bis(2-thienyl)acetate
[0284]
[0285] 2-Azaspiro[3.5]nonan-7-yl-2-hydroxy-2,2-bis(2-thienyl)acetate (2c) (0.1 g, 0.275 mmol) was dissolved in 10 ml of acetonitrile, Add 4-(5-formylbenzotriazol-1-yl)propyl methanesulfonate (see WO2017114377 for the synthesis method) (0.077g, 0.275mmol) and diisopropylethylamine (0.071g, 0.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com