Hepatitis b immunisation regimen and compositions
A kind of technology of hepatitis B, chronic hepatitis B, applied in a method and composition used in such scheme and method, the field of immunization scheme for the treatment of chronic hepatitis B
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
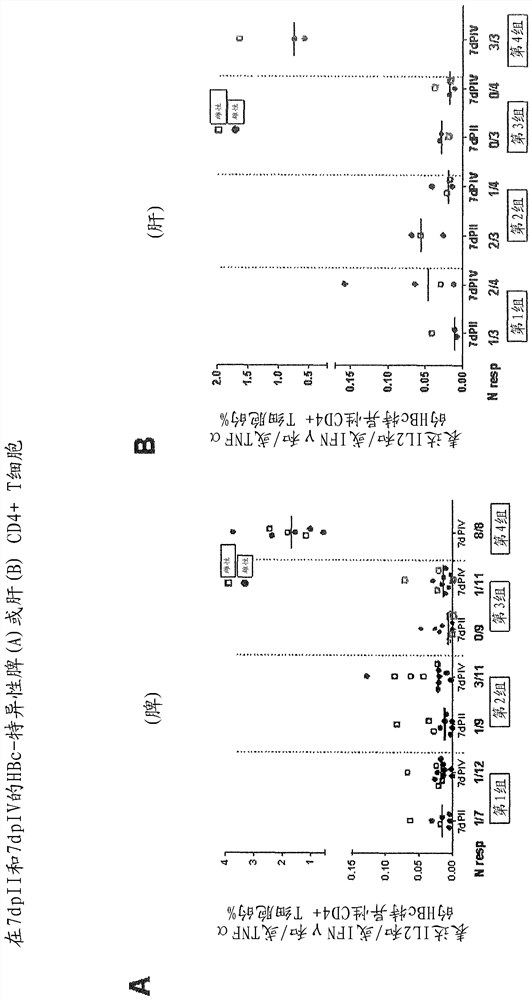
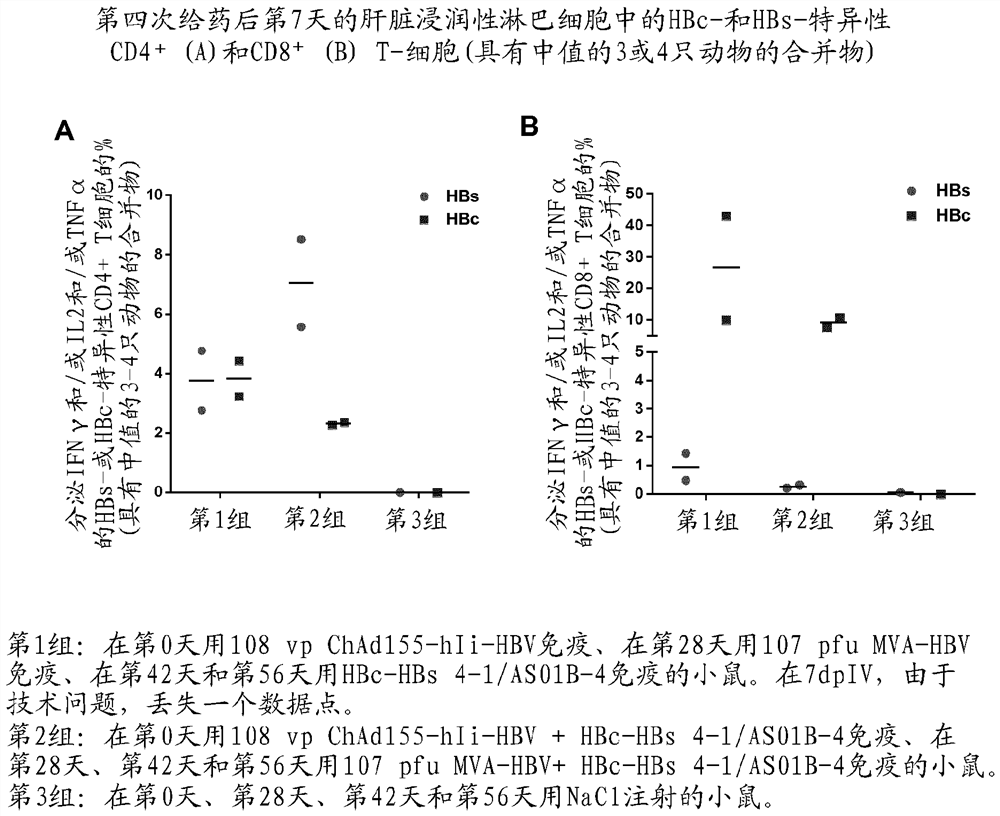
[0280] Example 1 - Evaluation of ChAd155-HBV (with and without hIi) priming in the HLA.A2 / DR1 transgenic mouse model and MVA-HBV enhanced
[0281] Target
[0282] The primary objective of this experiment was to determine whether priming with a dose of ChAd155-HBV (with or without hIi), followed by a booster dose of MVA-HBV, could induce the expression of a new gene in HLA.A2 / DR1 mice transgenic for human MHC-I / II molecules. Induces strong CD8 against HBc + T cell response. In addition, a head-to-head comparison between ChAd155-HBV with and without hli was performed to investigate that the hli sequence further increases HBc-specific CD8 + Potential for T-cell responses, as previously reported for other antigens [Spencer, 2014; Capone, 2014]. HBs-specific CD8 was also assessed + T-cell responses and HBc- and HBs-specific CD4 + T-cell and antibody responses.
[0283] Research design
[0284] HLA.A2 / DR1 mice (11 mice per group) were treated with 10 intramuscularly o...
Embodiment 2
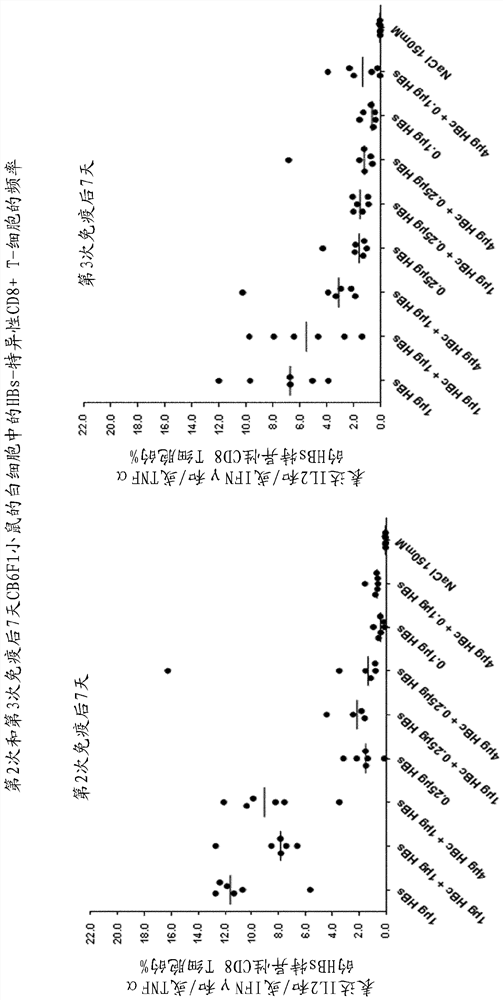
[0302] Example 2 - HBc-HBs / AS01 in inbred mice (CB6F1) B-4 Immunogenicity studies
[0303] Target
[0304] The main objective of this immunogenicity study was to determine when in AS01 B-4 Whether HBc and HBs proteins can induce HBc- and HBs-specific humoral and T-cell responses when co-formulated in .
[0305] Research design
[0306] 6- to 8-week-old CB6F1 mice (30 mice per group) were treated with 50 µl AS01 on days 0, 14 and 28 B-4 The HBc, HBs or HBc-HBs (listed in the following table 2) prepared in the Chinese medicine were immunized intramuscularly three times. HBc- and HBs-specific T cell responses were measured on fresh PBL 7 days after the second and third dose, and anti-HBs and anti-HBc antibodies were measured 14 days after the second and third dose answer.
[0307] Table 2: Treatment Groups
[0308] Group antigen 1 1 µg HBc / AS01 B-4 (HBc / AS01 B-4 )
2 1 µg HBs / AS01 B-4 (HBs / AS01 B-4 )
3 1µg HBc + 1µg HBs / AS01 B-4...
Embodiment 3
[0319] Example 3 - Comparative Adjuvant Experiments in Inbred Mice (CB6F1)
[0320] Target
[0321] The main purpose of this experiment was to compare the 4:1 ratio with different adjuvants (alum, AS01 B-4 or AS01 E-4 ) HBc and HBs antigens formulated together or without adjuvant induced strong CD4 against both antigens + Capacity for T-cell and humoral responses.
[0322] Research design
[0323] CB6F1 mice aged 6 to 8 weeks (35 mice for groups 1-4 and 25 mice for group 5) were treated with alum, AS01 B-4 or AS01 E-4 The prepared HBc-HBs antigen (4µg-1µg) (listed in Table 3 below) was immunized intramuscularly three times. with AS01 B-4 Compared to AS01 E-4 The adjuvant system contains half the amount of immune enhancers QS-21 and MPL. HBc- and HBs-specific T cell responses were measured on fresh PBL 7 days after the second and third doses, after ex vivo restimulation with the pool of peptides for 6 hours, and after the second and third doses Anti-HBs and anti-HB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com