Modified T cells and uses thereof
A cell, selected technology, applied in the field of T cells, can solve problems such as harming the favorable results of critically ill patients, infeasible preparation of autologous products, and inability to immediately administer drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1. TCR / CD3 Complex Disruption in CAR T Cells
[0047] Sequences encoding sgRNAs targeting TCRα, CD247ζ, CD3ε, CD3γ and CD3δ driven by T7 promoter were cloned into BH-MSG vector respectively.
[0048] Cas9 and sgRNA plasmids were linearized prior to RNA in vitro transcription (IVT). Store IVT RNA in nuclease-free vials at -80°C for single use. Cas9 mRNA was transcribed in vitro with the mMESSAGE mMACHINE T7ULTRA kit (LifeTechnologies, AM1345, Carlsbad, CA). sgRNA was transcribed with HiScribeTM T7 High YieldRNA Synthesis Kit (NEB).
[0049] To prepare modified CAR T cells, primary human CD4 and CD8 T cells were isolated from healthy volunteer donors by leukapheresis via Ficoll-PaqueTM PREMIUM (GE healthcare). Then, T cells were first activated with CD3 / CD28 magnetic beads, and then transduced with CAR-expressing lentivirus. Afterwards, CAR T cells were washed 3 times with OPTI-MEM and resuspended in OPTI-MEM (Invitrogen) at a final concentration of 1–3×10 8 ...
Embodiment 2
[0055] Example 2. Expression of TCR / CD3 complexes in modified CAR T cells
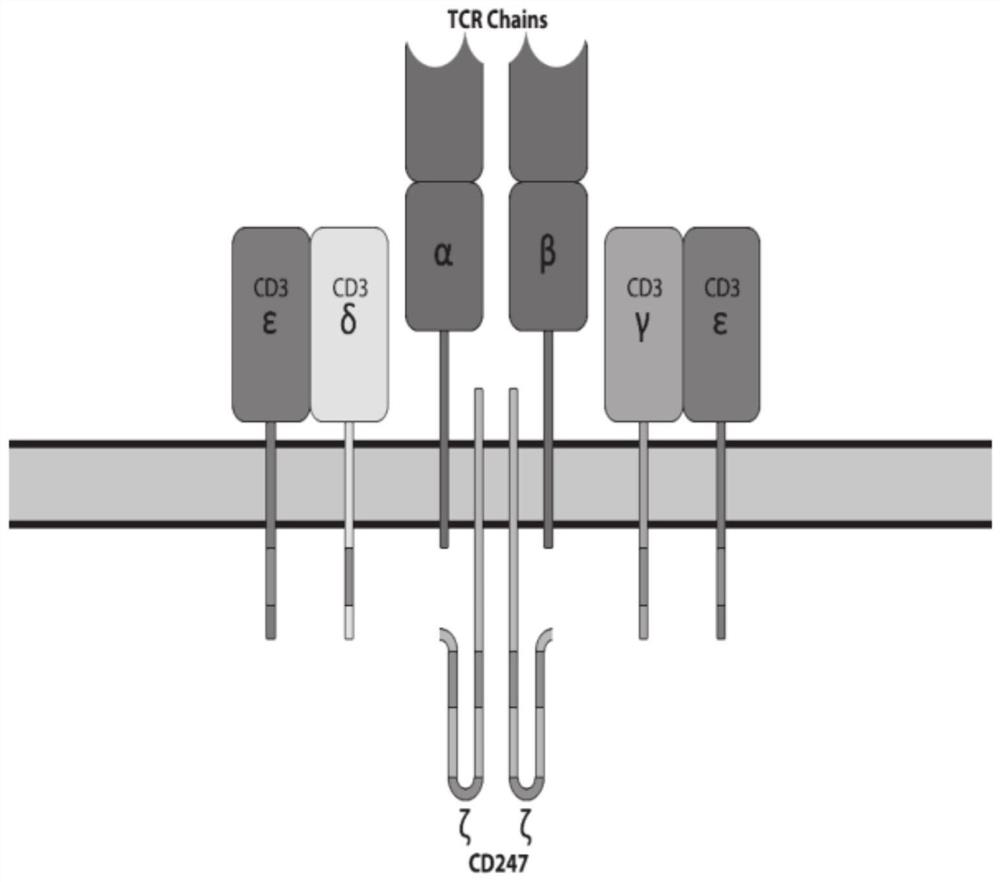
[0056] Disruption of TCR components (α or β chains) in α / β T cells has been reported to result in loss of TCR / CD3 complex expression, thereby preventing GvHD effects of allogeneic T cells. We hypothesized that the integrity of the TCR / CD3 complex requires the complete assembly of TCR components, CD3 components and CD247ζ.
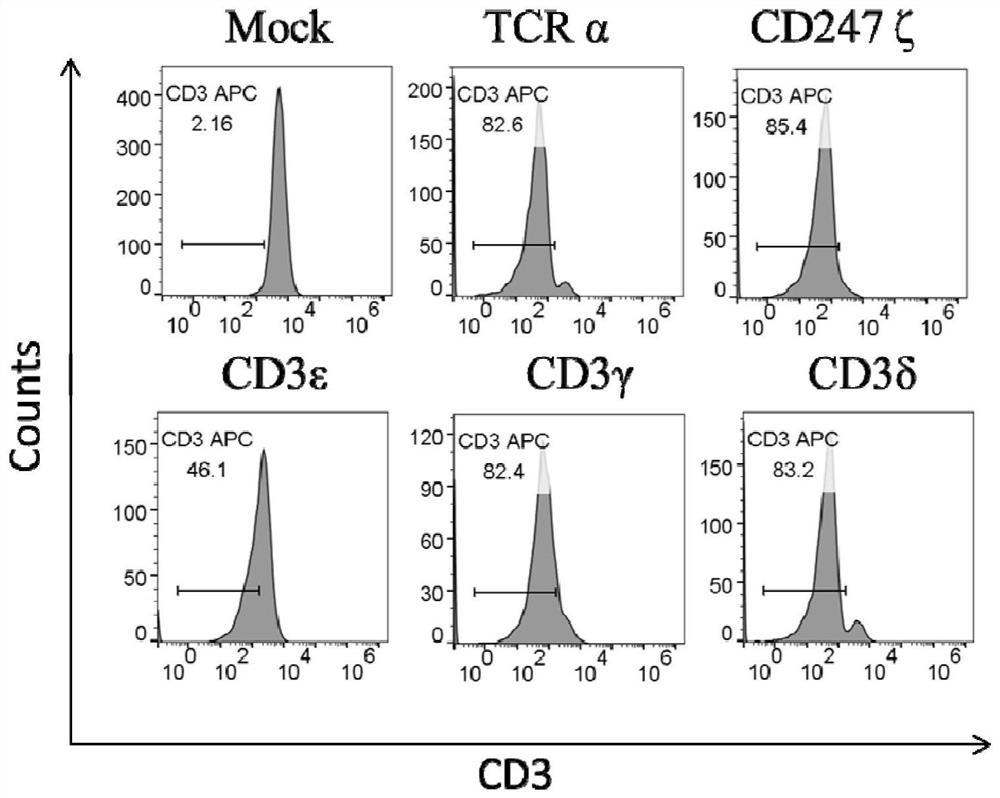
[0057] Therefore, to test whether disruption of CD3 components and CD247ζ might lead to disruption of the TCR / CD3 complex, we knocked out CD3 components (including CD3γ, CD3δ, and CD3ε) and CD247ζ by CRISPR gene editing.
[0058] Modified CAR T cells were obtained according to the method described in Example 1. The genomic DNA of the modified CAR T cells was extracted, and the PCR products flanking the target site were subjected to Sanger sequencing to confirm the target editing on the DNA strand. Results were also analyzed by TIDE (Tracking Indel, DE composition) software. Genom...
Embodiment 3
[0061] Example 3. Determining the Phenotype of Modified CAR T Cells
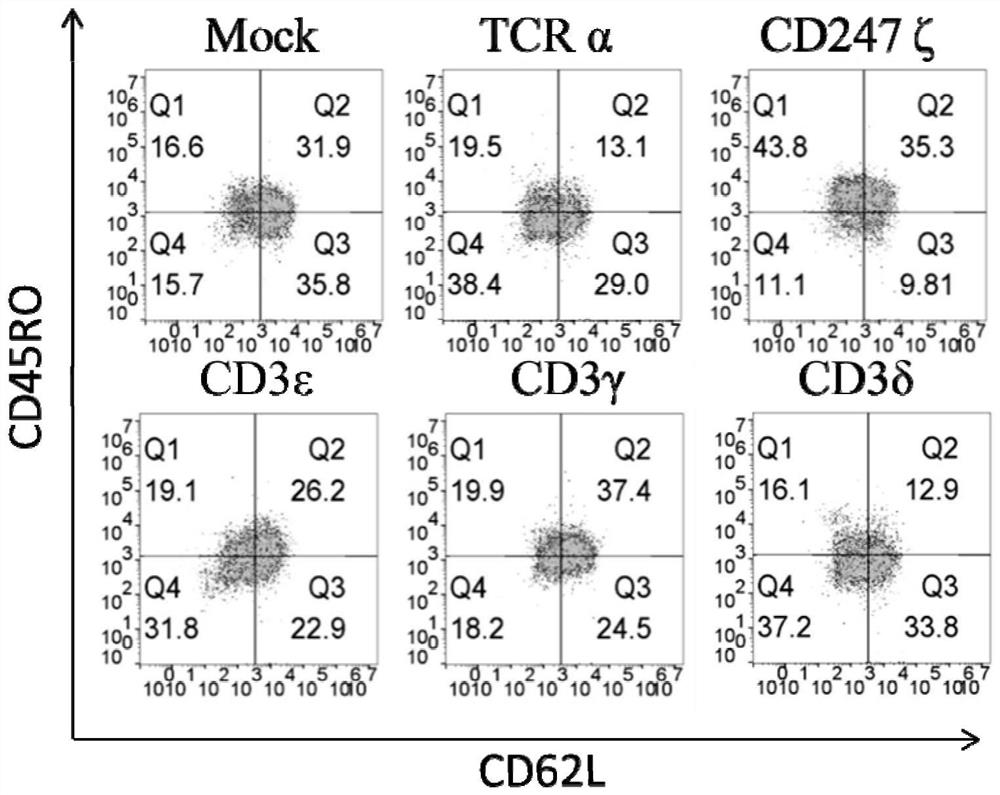
[0062] To measure the CD3 component and the central memory phenotype of CD247ζ-disrupted T cells, the expression of CD45RO and CD62L was determined by flow cytometry. The result is as image 3 shown.
[0063] Surprisingly, we found that CD247ζ, CD3ε, and CD3γ knockout CAR T cells exhibited a more CD45RO and CD62L double-positive central memory phenotype than TCRα knockout CAR T cells. Specifically, there were approximately 31.9% CD45RO and CD62L double positive central memory cells in the simulated T cell population. In TCRα knockout cells, the CD45RO and CD62L double-positive cell population was reduced to 13.1%. However, CD247ζ, CD3ε, and CD3γ-disrupted T cells contained higher numbers of CD45RO and CD62L double-positive central memory cells at levels comparable to mock T cells, compared with TCRα knockout. It was also noted that CD3δ knockdown resulted in a central memory phenotype comparable to that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com