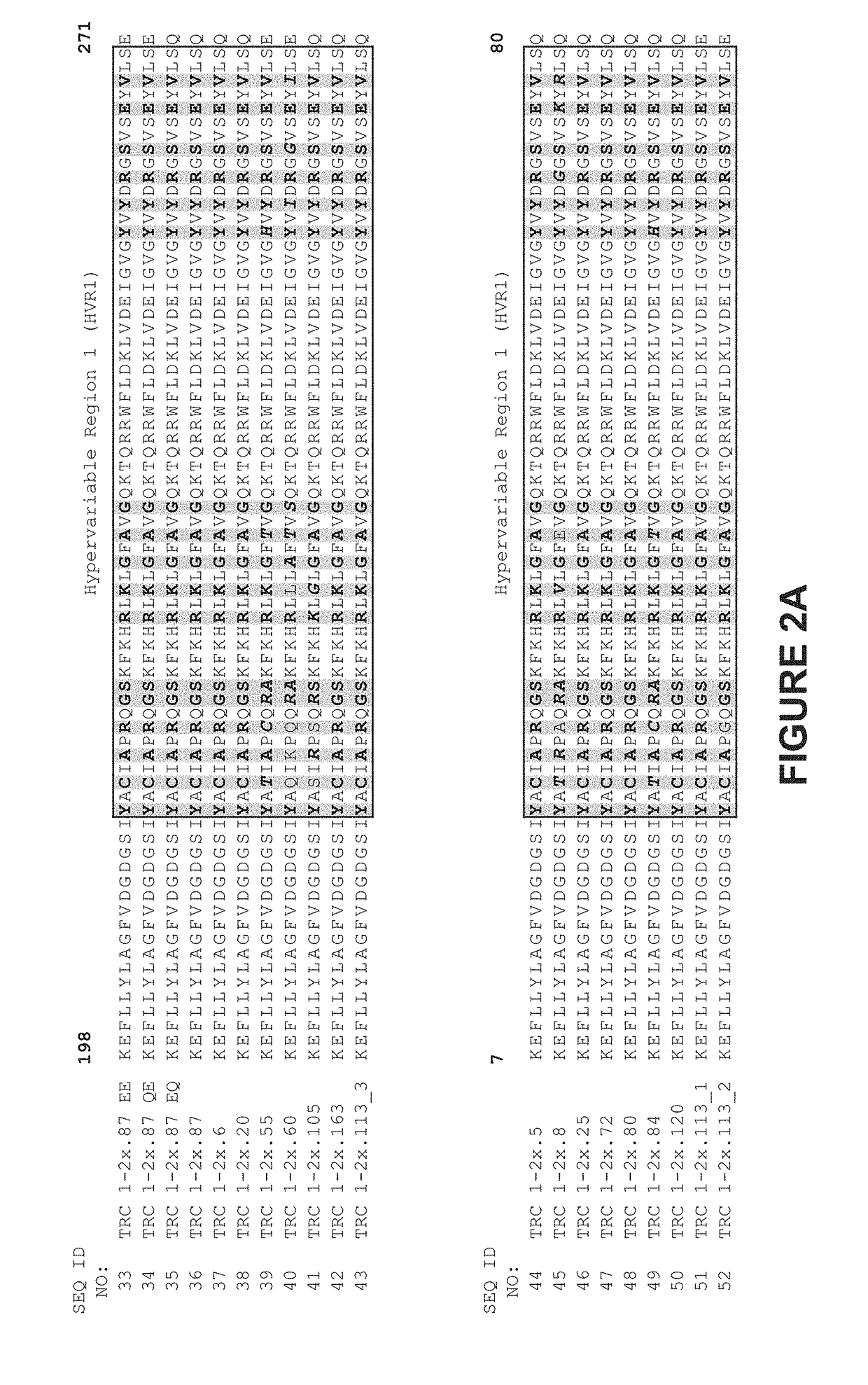
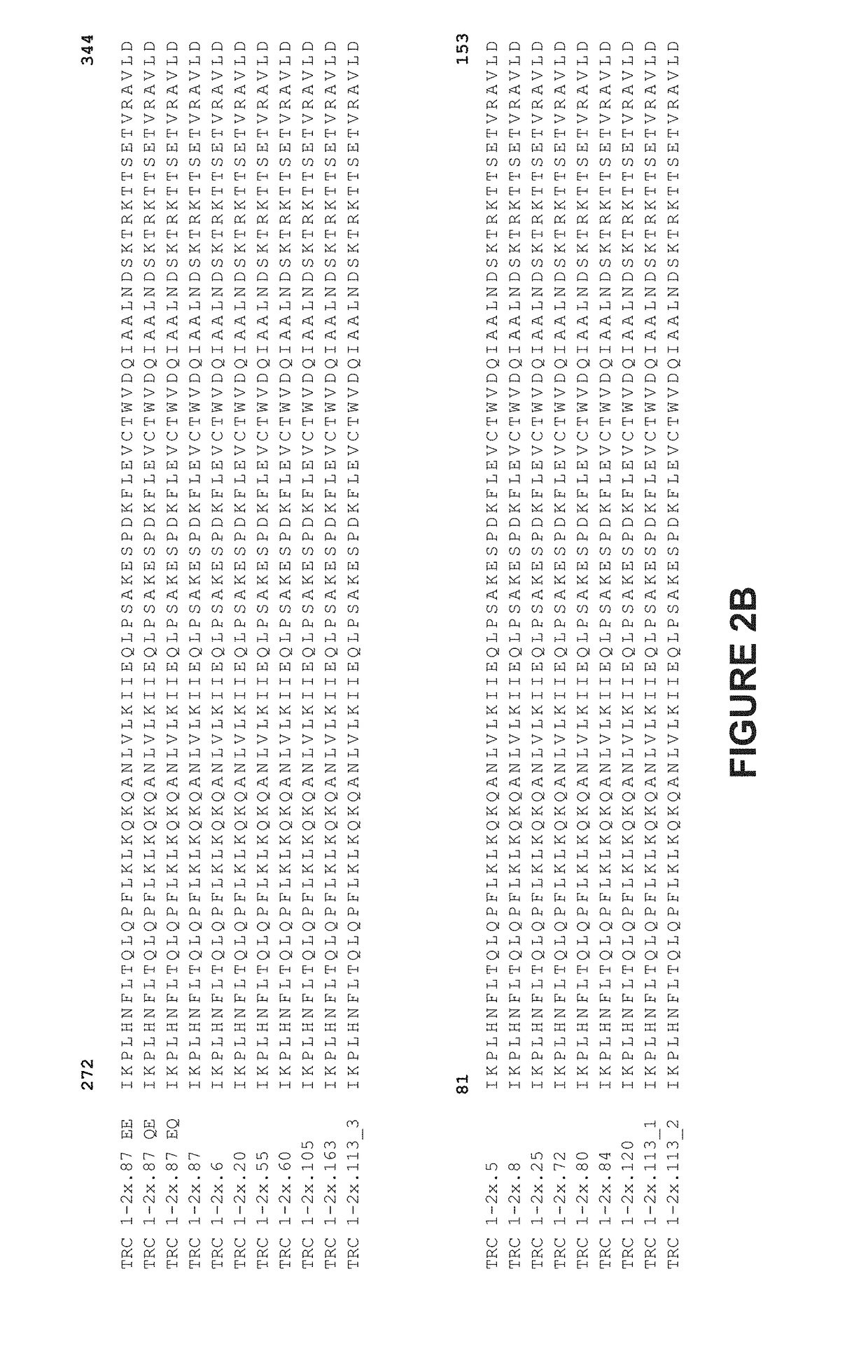
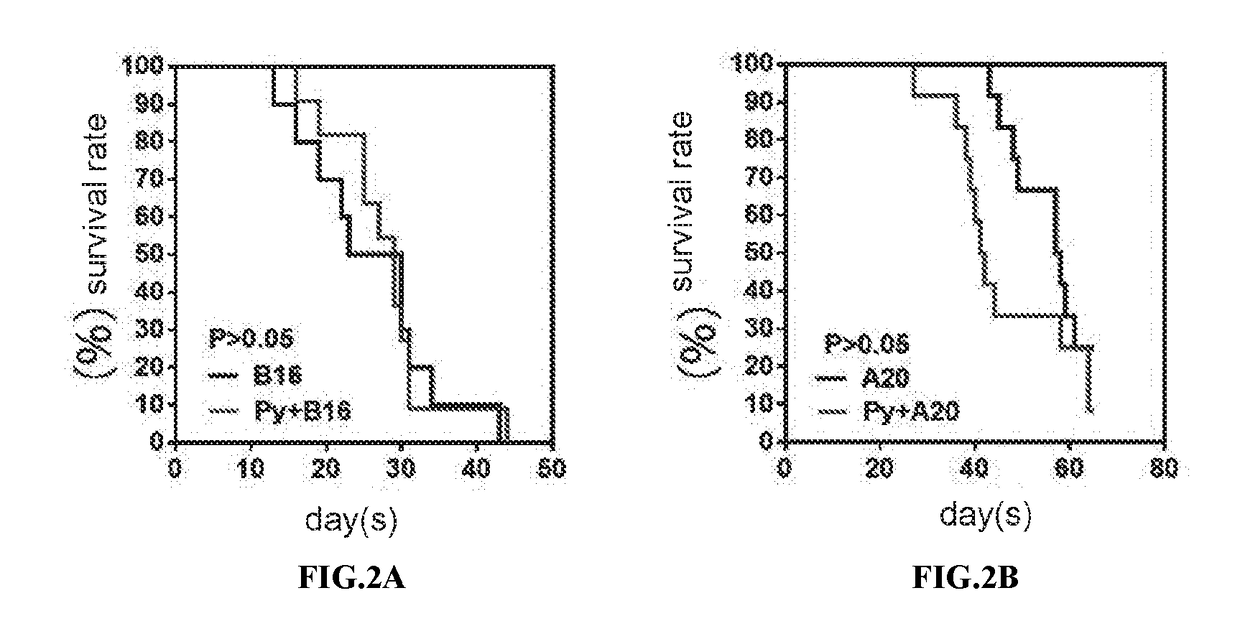
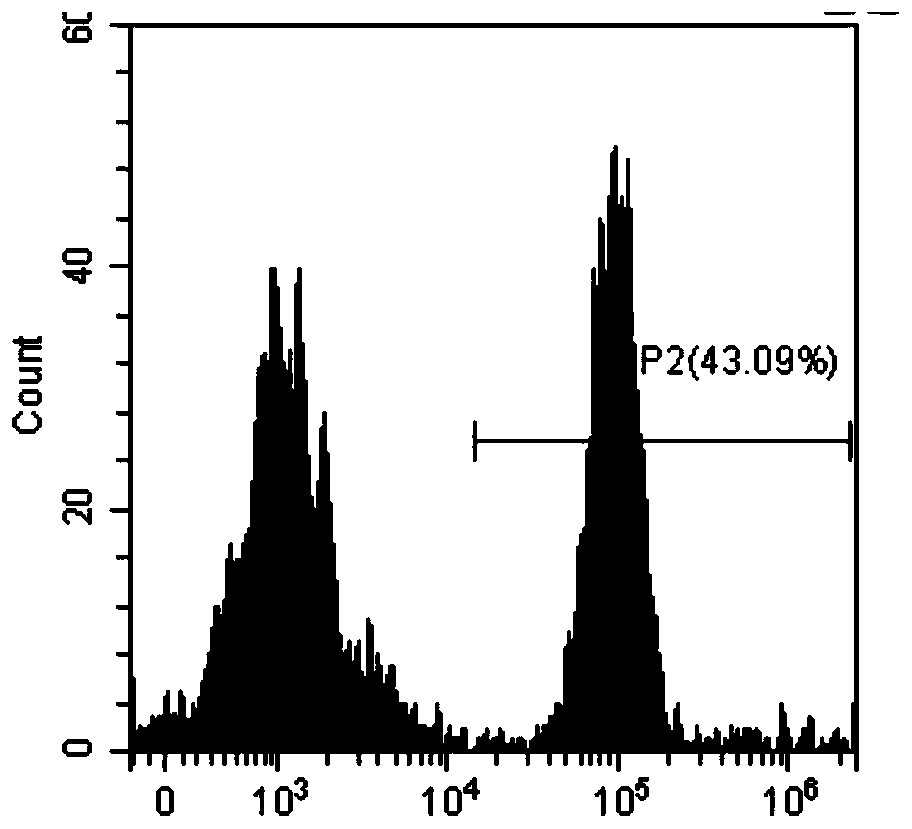
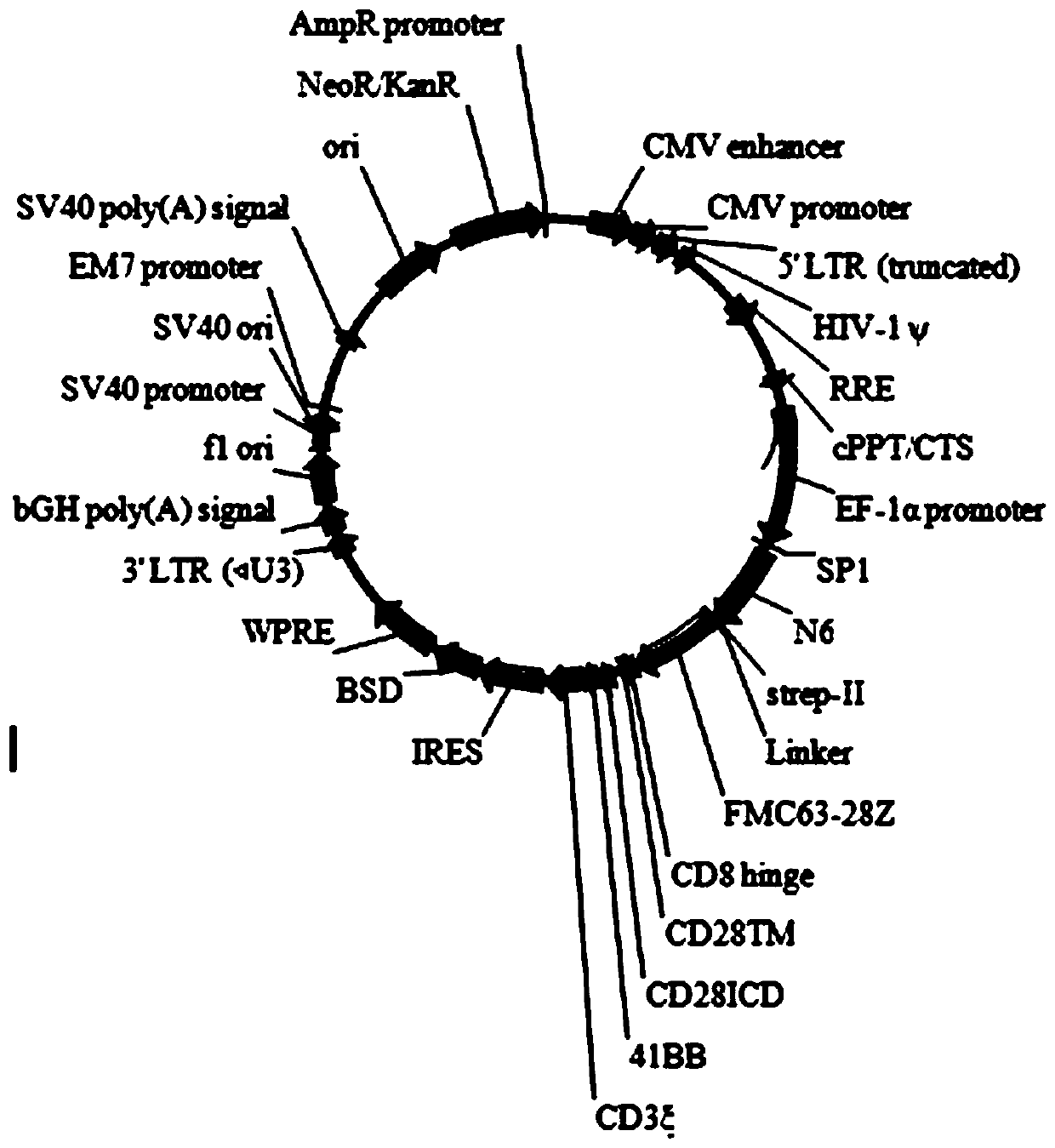
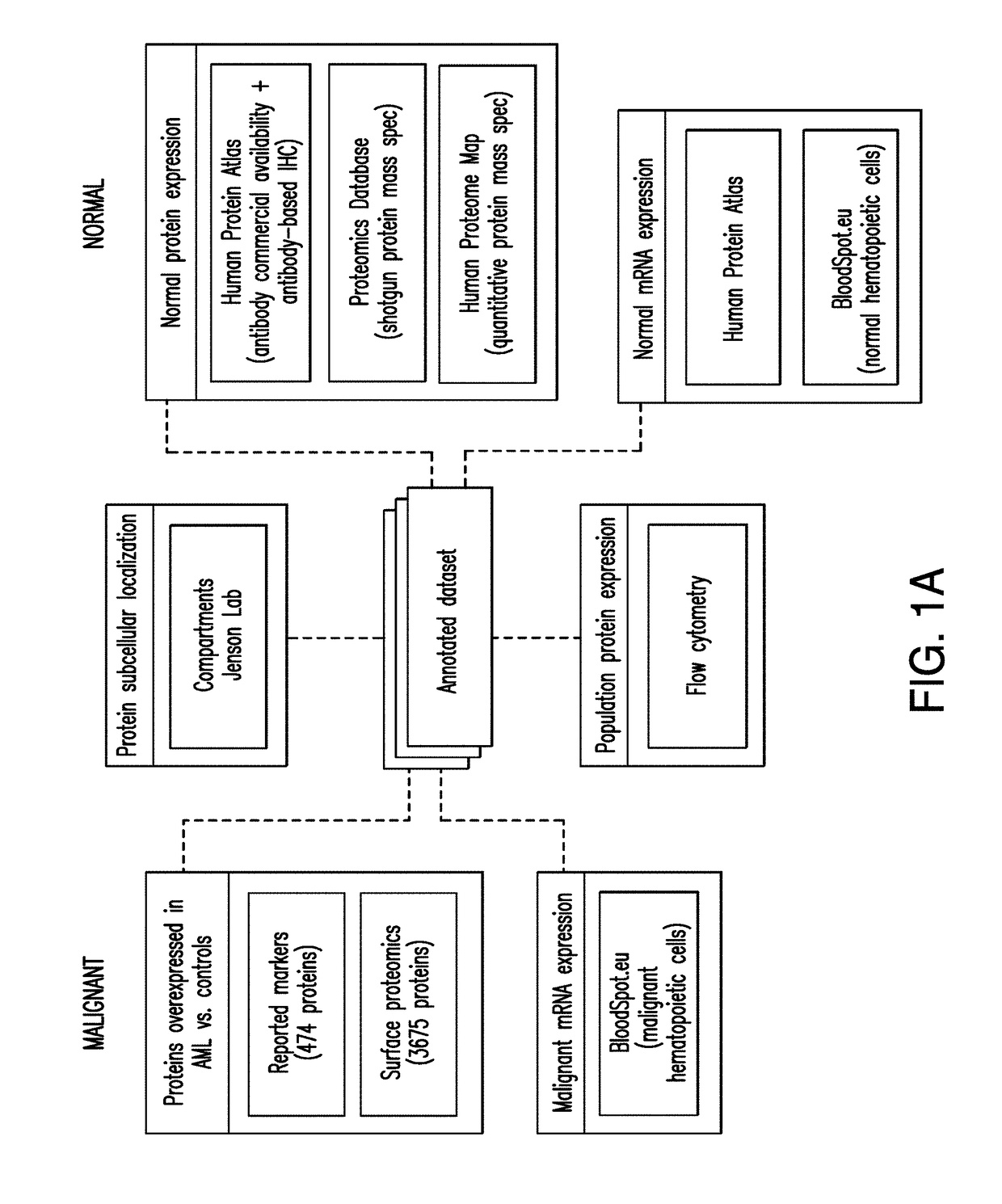
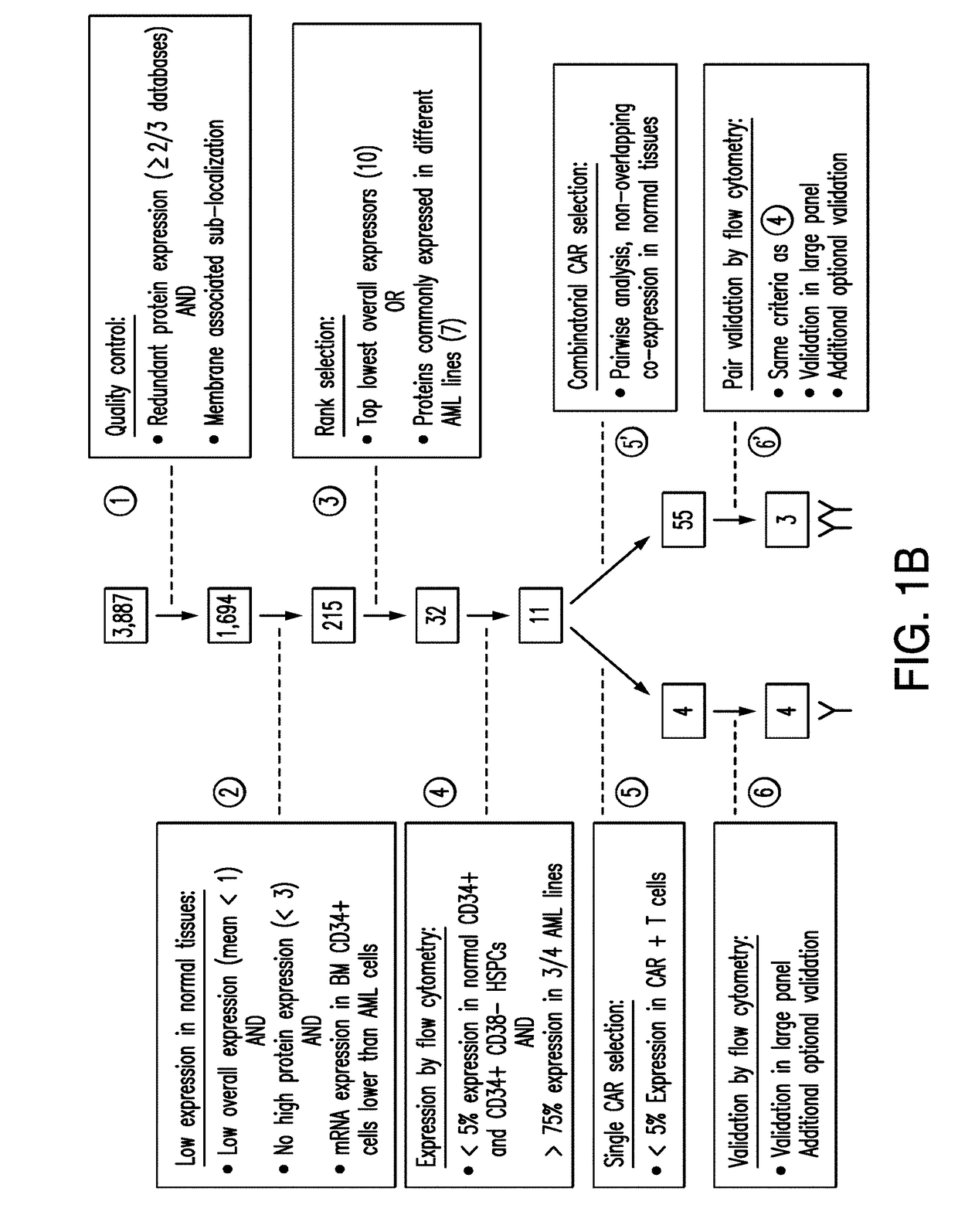
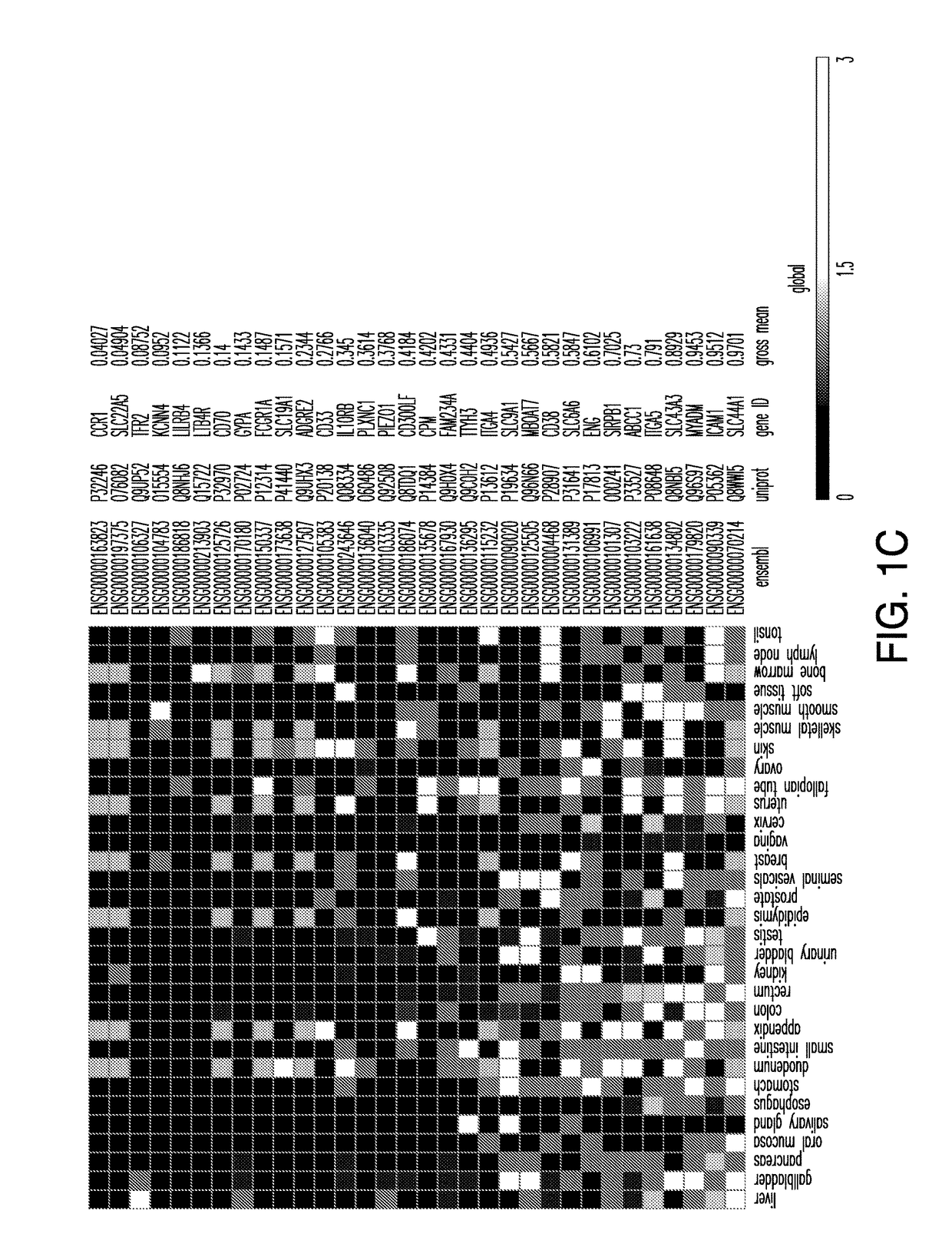
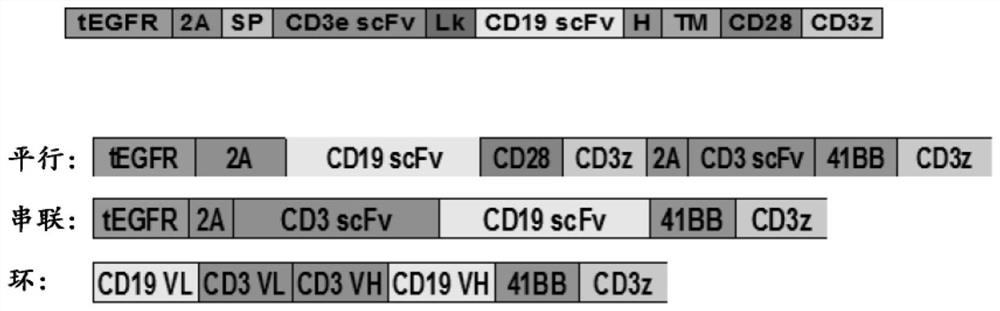
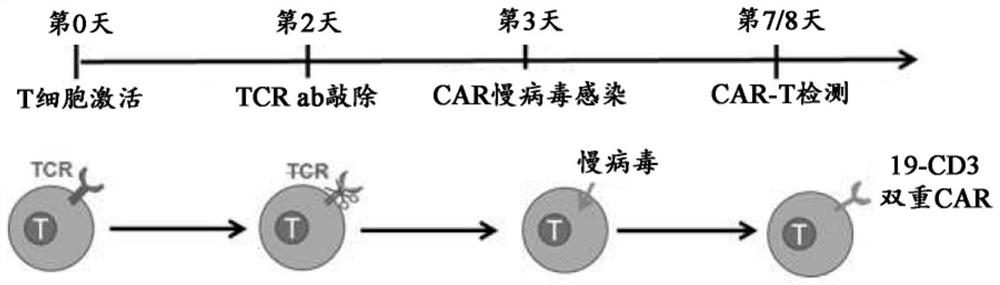
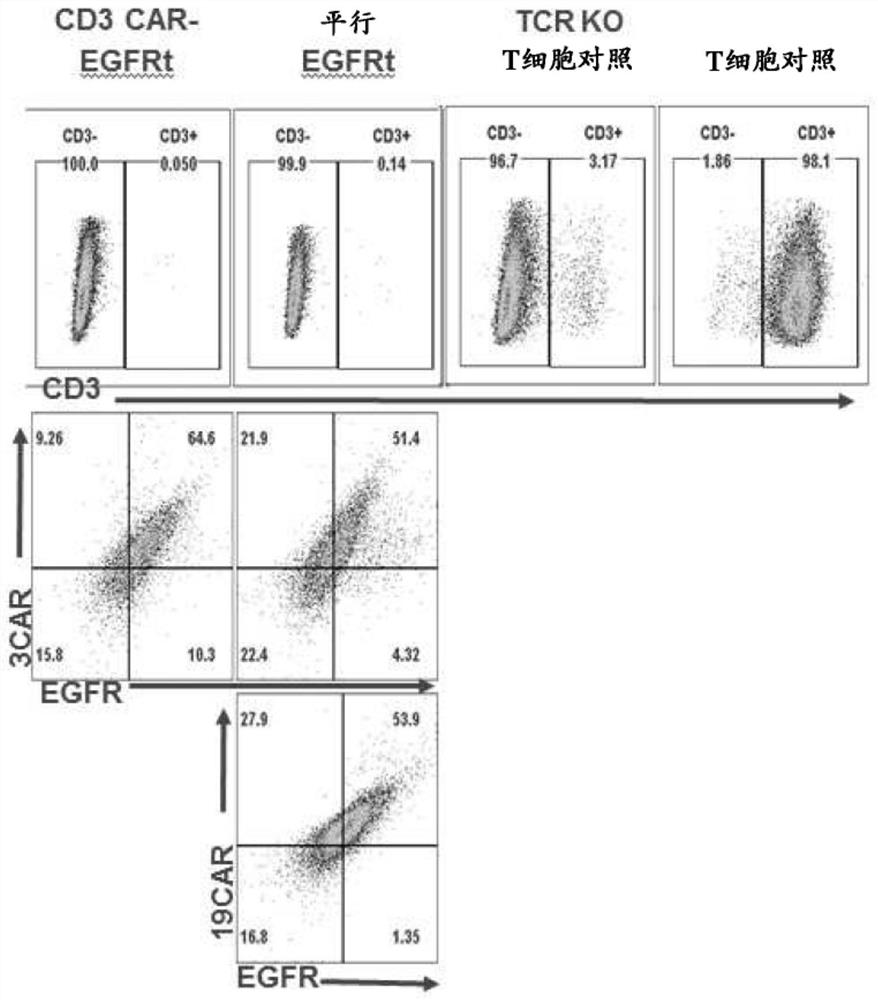
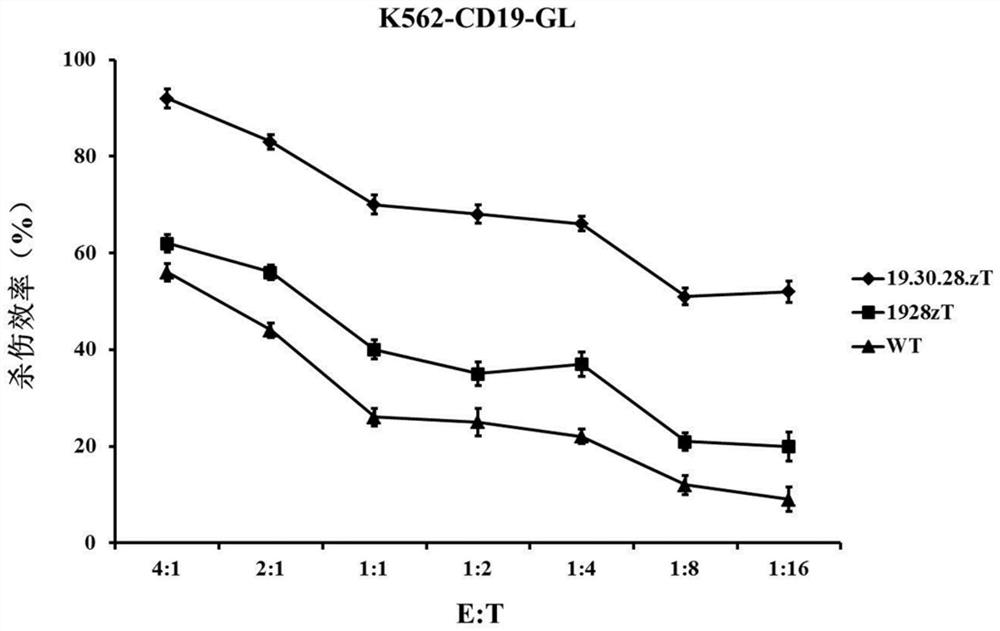
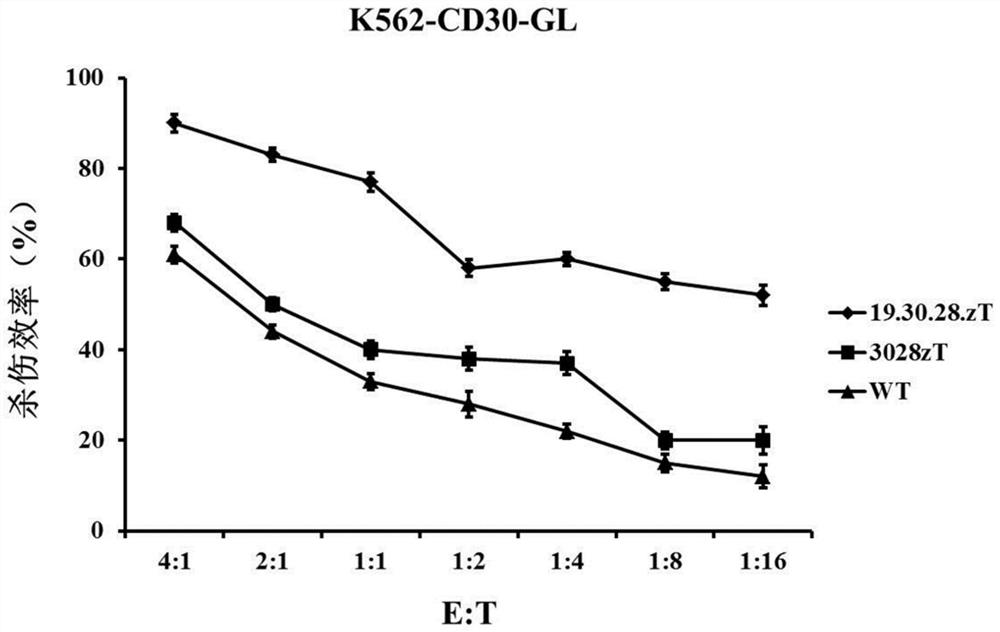
Patents
Literature
256results about "Blood cell cancer vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
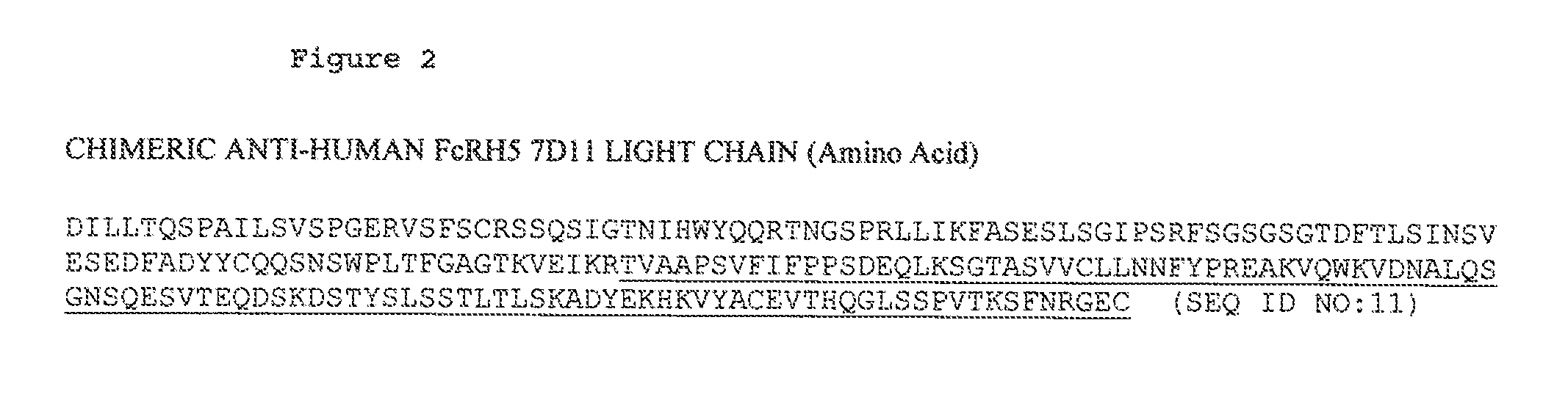
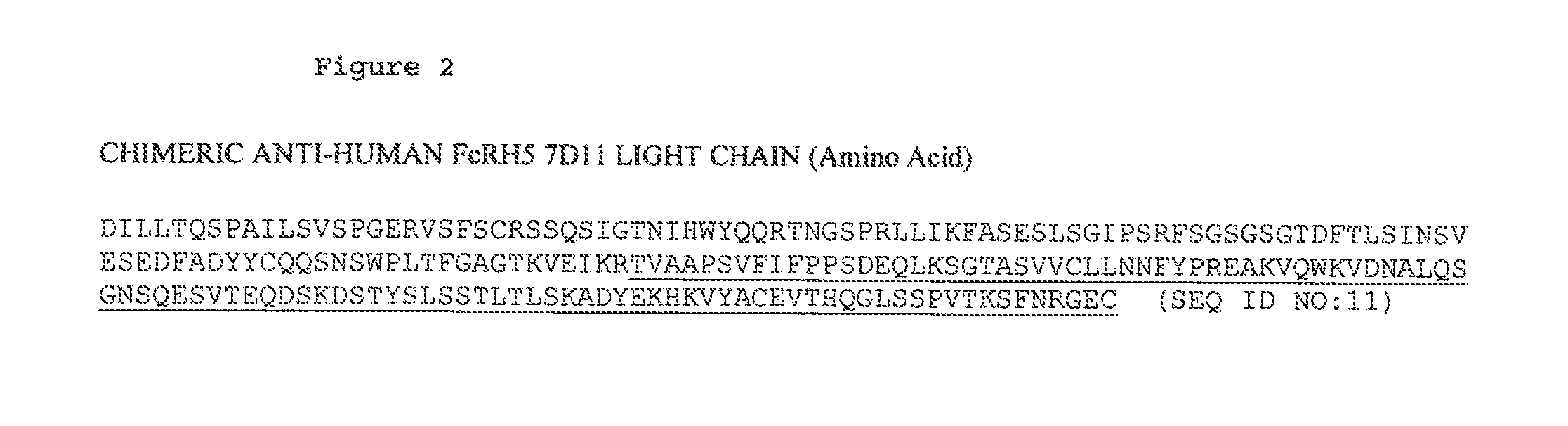
ANTI-FcRH5 ANTIBODIES AND IMMUNOCONJUGATES AND METHODS OF USE
InactiveUS20100260748A1Heavy metal active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesBiochemistry
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:F HOFFMANN LA ROCHE & CO AG
Genetically-modified cells comprising a modified human T cell receptor alpha constant region gene
ActiveUS9889160B2Efficient insertionConvenient treatmentAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseGene Modification
Disclosed herein is a genetically-modified cell comprising in its genome a modified human T cell receptor alpha constant region gene, wherein the cell has reduced cell-surface expression of the endogenous T cell receptor. The present disclosure further relates to methods for producing such a genetically-modified cell, and to methods of using such a cell for treating a disease in a subject.
Owner:PRECISION BIOSCI
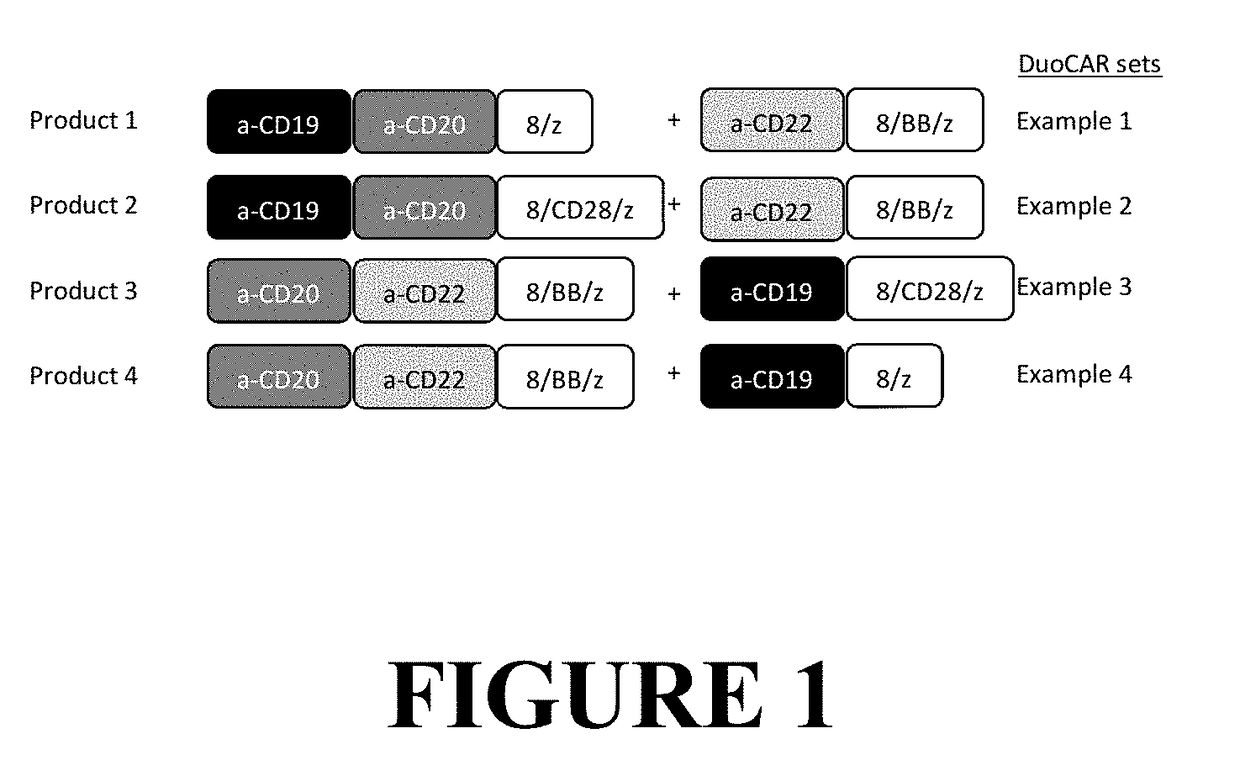
Compositions and Methods for Treating Cancer with DuoCARs
ActiveUS20190083596A1Prevent and ameliorate relapseAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsIntracellular signallingDisease
Novel therapeutic immunotherapy compositions comprising at least two vectors, each vector encoding a functional CAR, whereby the combination of vectors results in the expression of two or more non-identical binding domains, wherein each vector encoded binding domain(s) are covalently linked to a transmembrane domain and one or more non-identical intracellular signaling motifs are provided herein as well as are methods of use of same in a patient-specific immunotherapy that can be used to treat cancers and other diseases and conditions.
Owner:LENTIGEN TECH INC
CD19-based chimeric antigen receptor and application thereof
PendingCN108383914ALess prone to stormsGood killing effectPeptide/protein ingredientsAntibody mimetics/scaffoldsAbnormal tissue growthSide effect
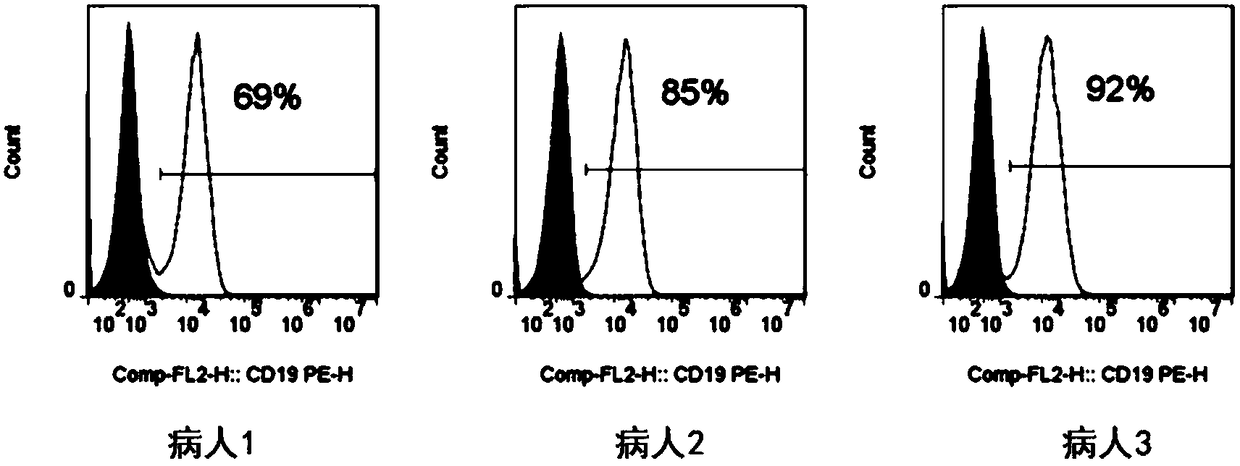
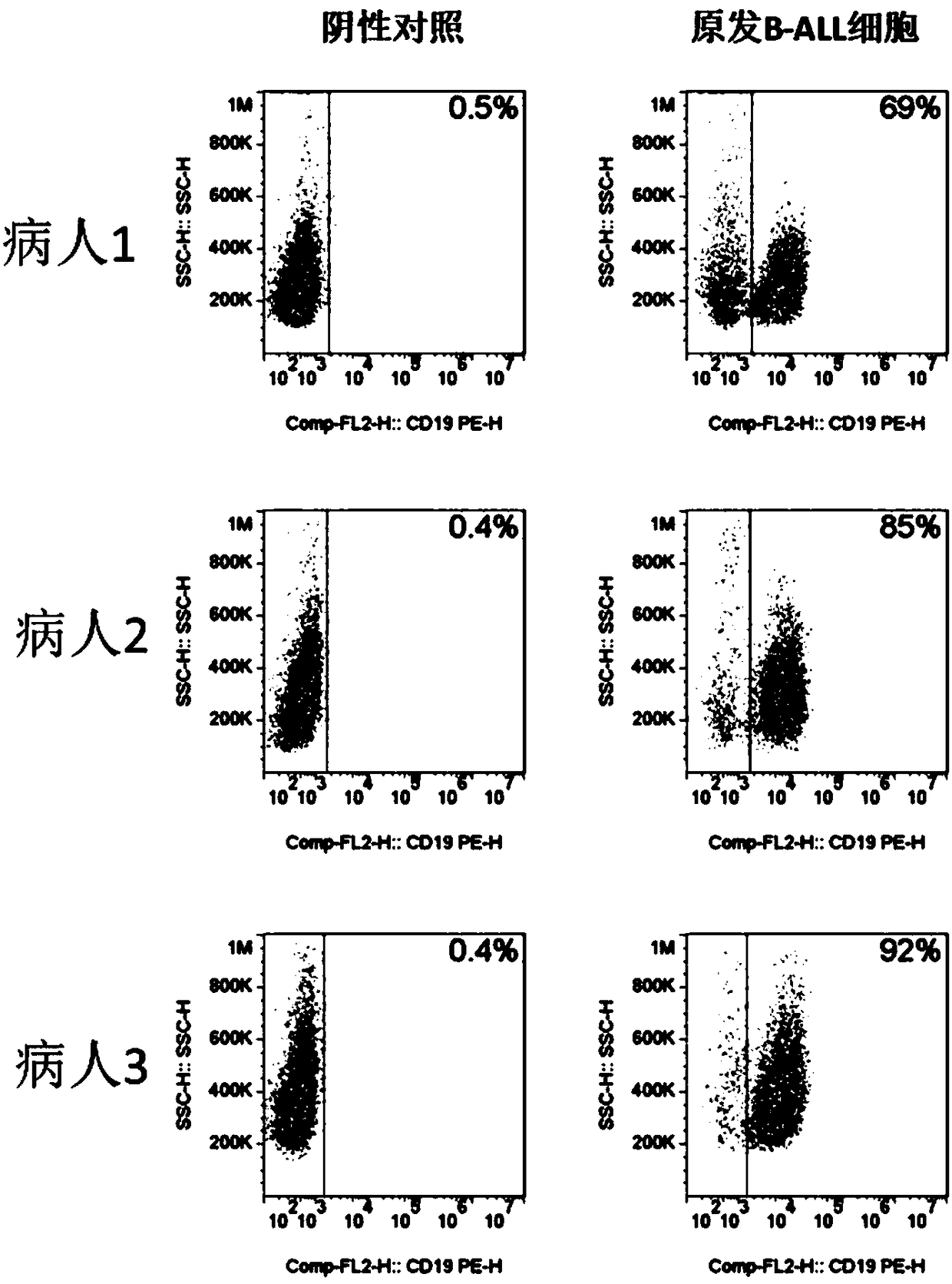
The invention relates to a CD19-based chimeric antigen receptor and application thereof, in particular to a lentivirus vector material built by a chimeric antigen receptor T (CAR-T) cell technology using a tumor specific target point CD19 as the basis, a method, and application thereof to anti-tumor treatment. The chimeric antigen receptor is formed by serially connecting an antigen combination structure domain, a membrane spaning structure domain, a costimulatory signal conduction region, a CD3 zeta signal conduction structure domain and an inducible suicide fusion structure domain, wherein the antigen combination structure domain is combined with the tumor surface antigen; the tumor surface antigen is CD19. The chimeric antigen receptor is subjected to specific gene transformation on theT cell stimulation signals. Compared with other chimeric antigen receptors, the chimeric antigen receptor provided by the invention has a better reaction effect and higher safety, so that the CAR-T cells have higher immune effects and low side effects; the treatment effect and safety of the CAR-T cells are enhanced.
Owner:BEIJING MEIKANG JIMIAN BIOTECH CO LTD
Expanded nk cells
InactiveUS20090123442A1Strong cytotoxicityGood effectBiocideCulture processNatural Killer Cell Inhibitory ReceptorsCytotoxicity
The present invention relates to expanded NK cells. The NK cells have been expanded ex vivo, are activated and have a cytotoxic phenotype. The cytotoxicity against malignant cells is markedly increased compared to non-expanded NK cells. The invention also relates to a method of treatment.
Owner:CELLPROTECT NORDIC PHARMA
Tumor cell vaccines
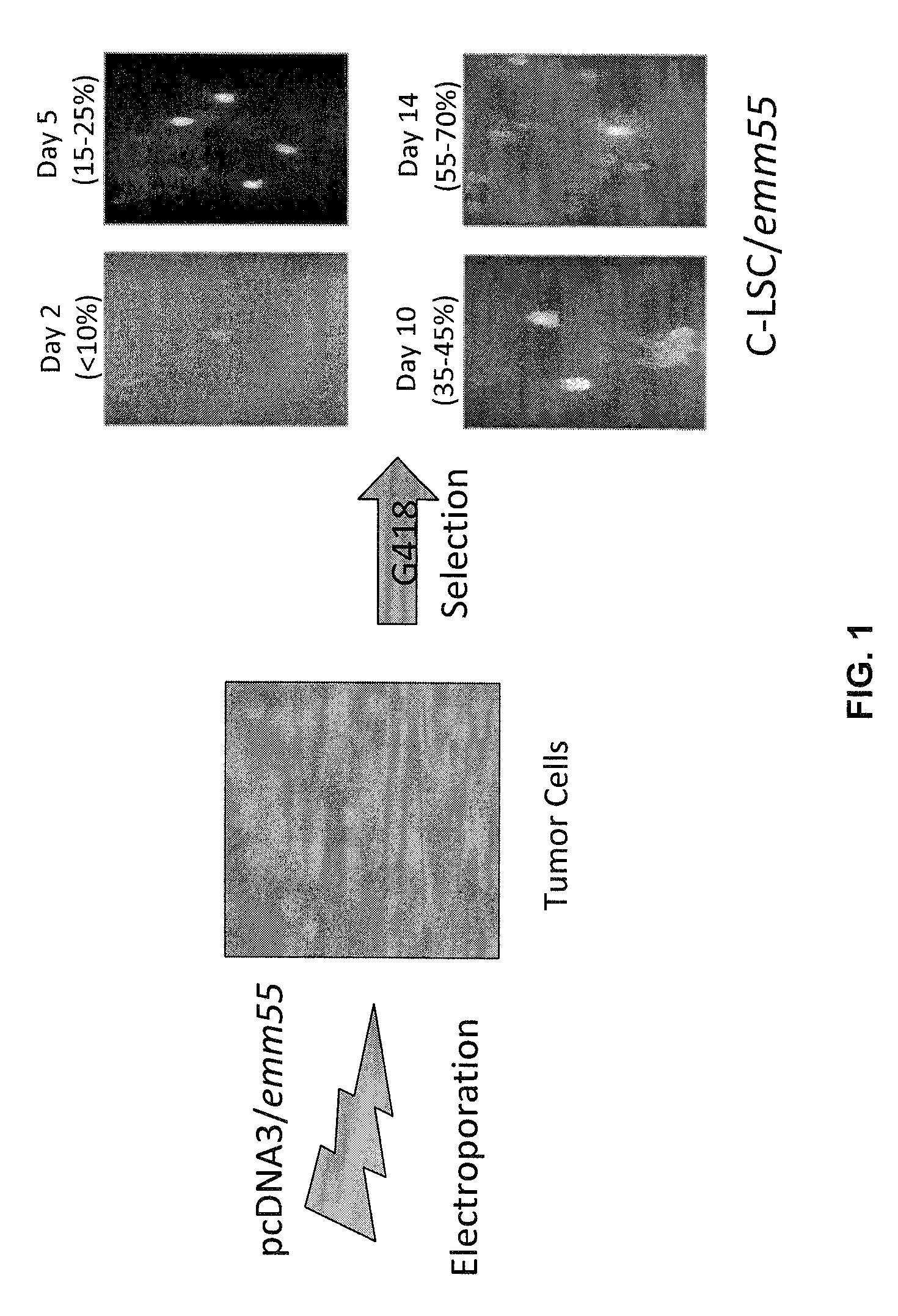
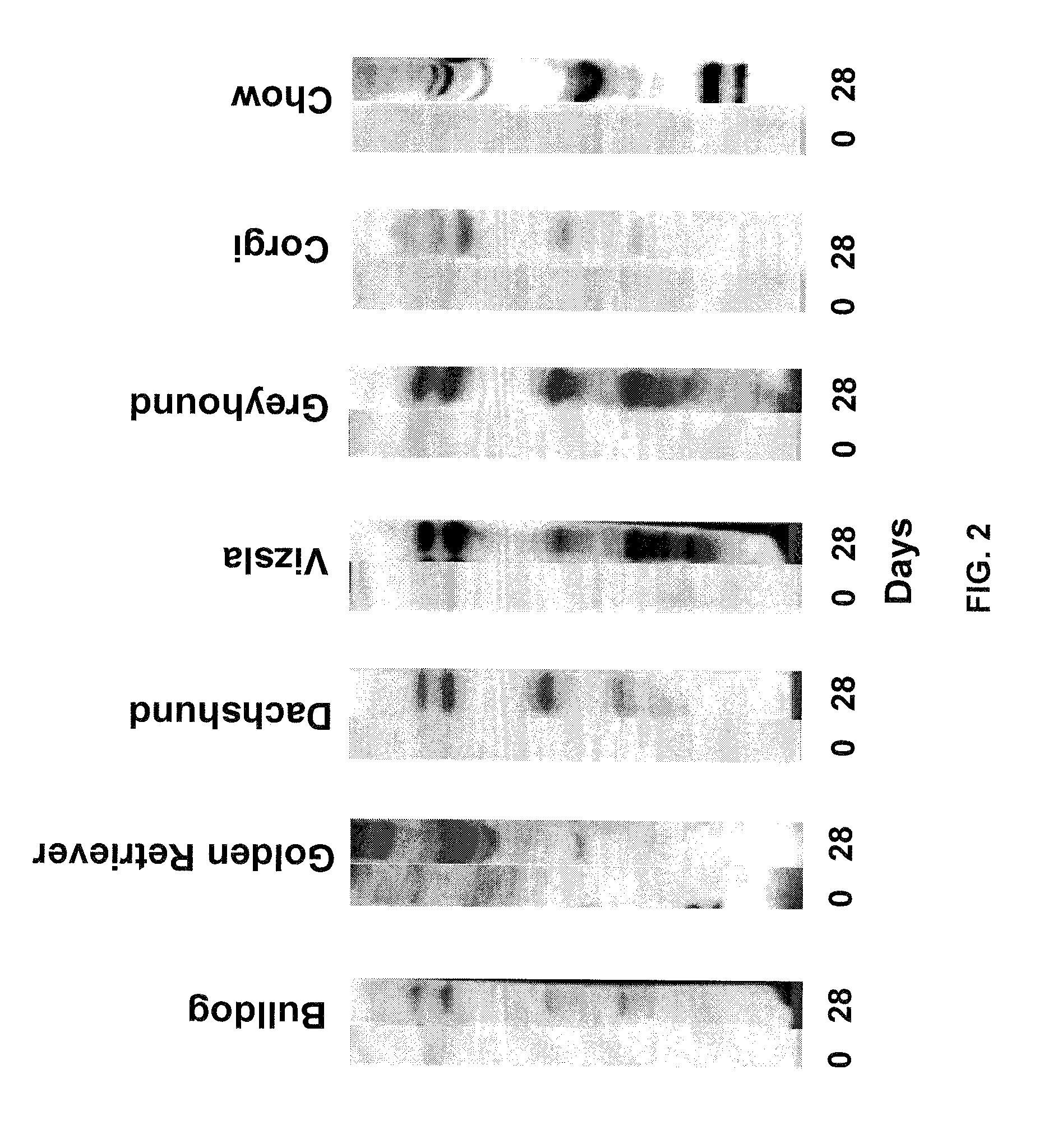
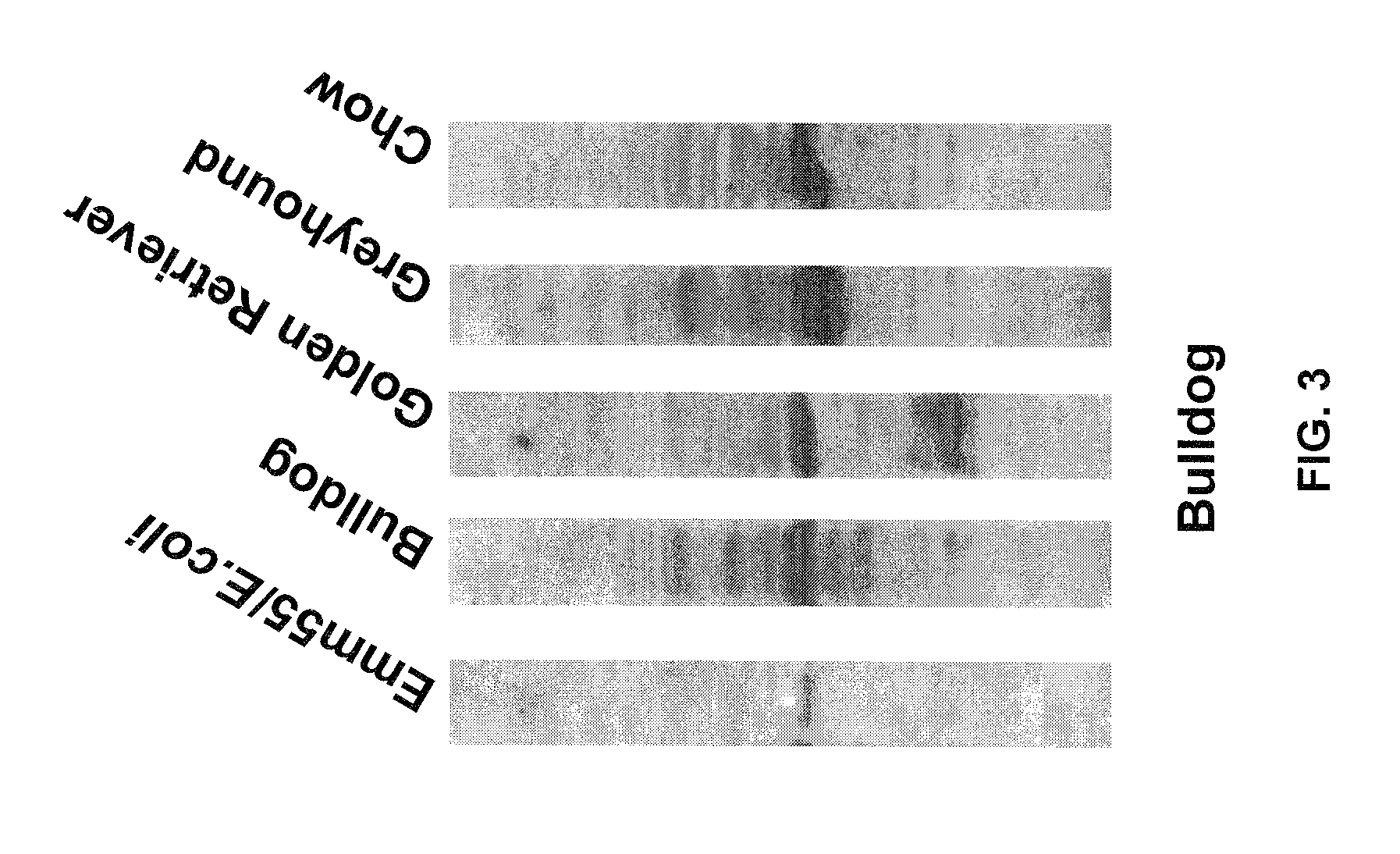
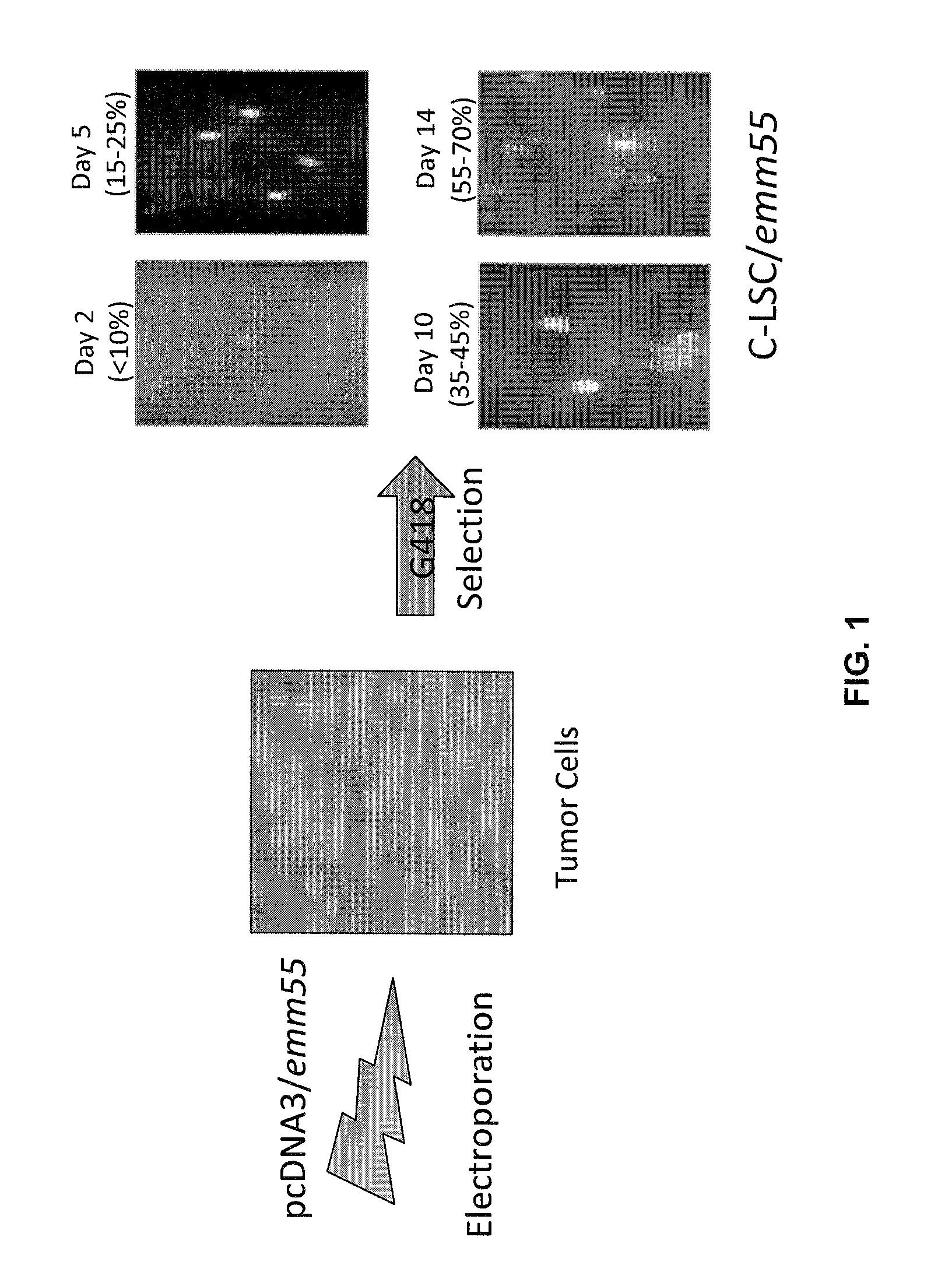
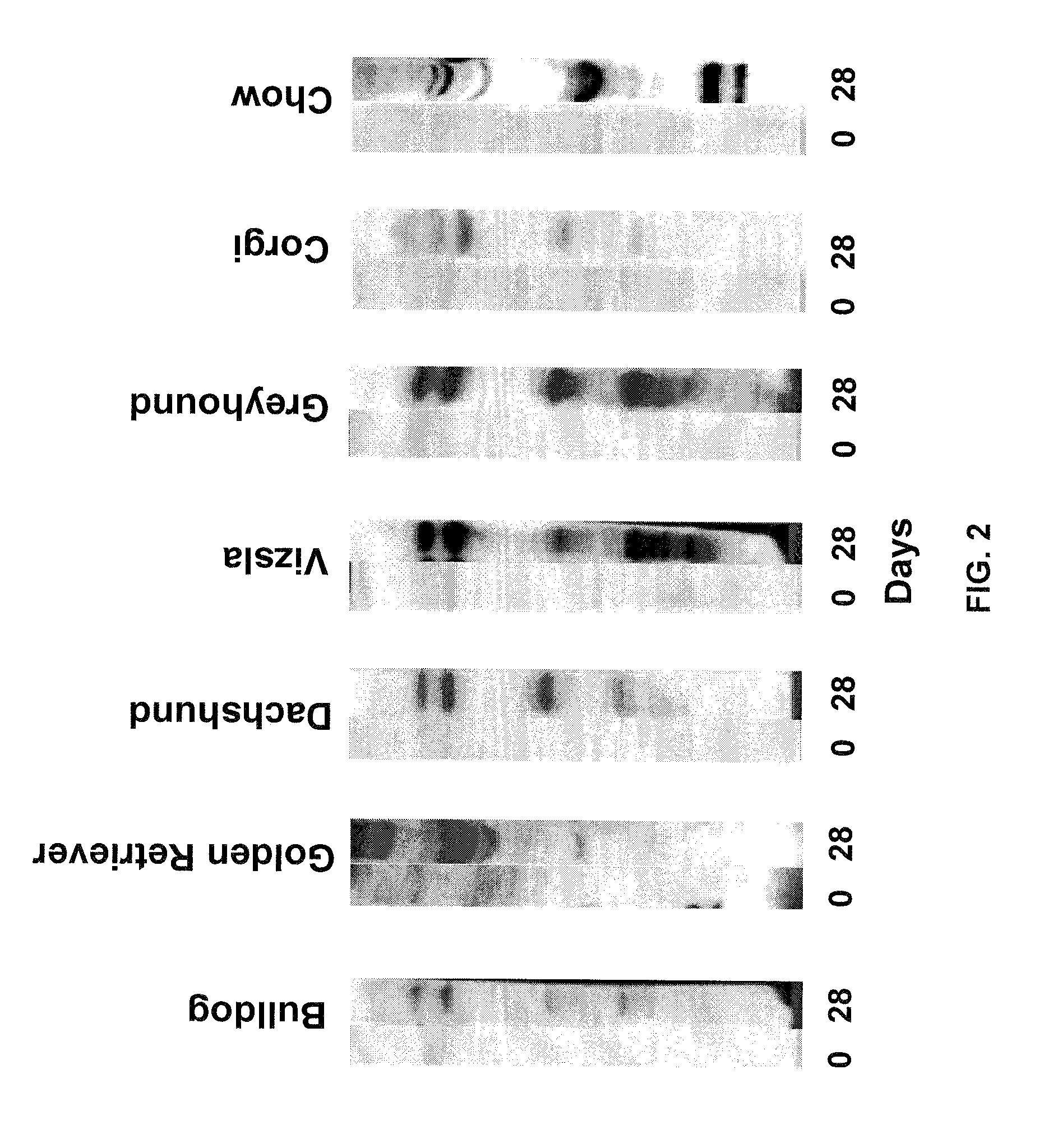
InactiveUS7795020B2Maximize potentialExtend your lifeBiocidePeptide/protein ingredientsCancer cellCanis lupus familiaris
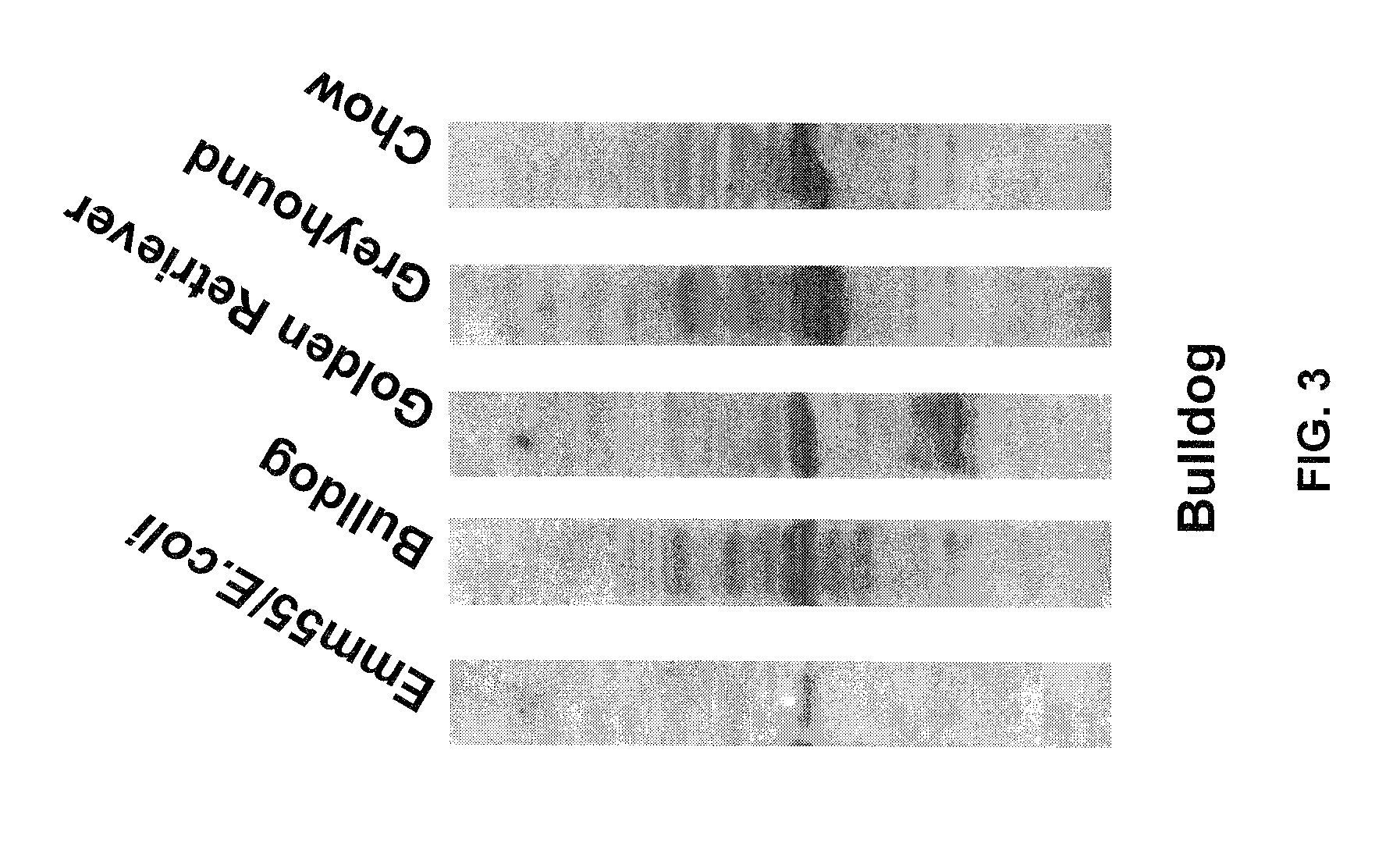
An effective cancer cell vaccine for canines has been developed. The vaccine is prepared from autologous lymphoma cells transfected with emm55. Once an animal is vaccinated, the expressed Emm55 antigen stimulates an immunogenic response to the tumor cells resulting in significantly increased survival, strong autologous and cross reactive humoral and cell mediated responses in several breeds of dogs diagnosed with later stage lymphomas.
Owner:MORPHOGENESIS
Genetically-modified cells comprising a modified human T cell receptor alpha constant region gene
ActiveUS9889161B2Efficient insertionConvenient treatmentAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseT-Cell Receptor Alpha
Owner:PRECISION BIOSCI
Genetically-modified cells comprising a modified human t cell receptor alpha constant region gene
ActiveUS20170333481A1Efficient insertionConvenient treatmentAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseT-Cell Receptor Alpha
Disclosed herein is a genetically-modified cell comprising in its genome a modified human T cell receptor alpha constant region gene, wherein the cell has reduced cell-surface expression of the endogenous T cell receptor. The present disclosure further relates to methods for producing such a genetically-modified cell, and to methods of using such a cell for treating a disease in a subject.
Owner:PRECISION BIOSCI
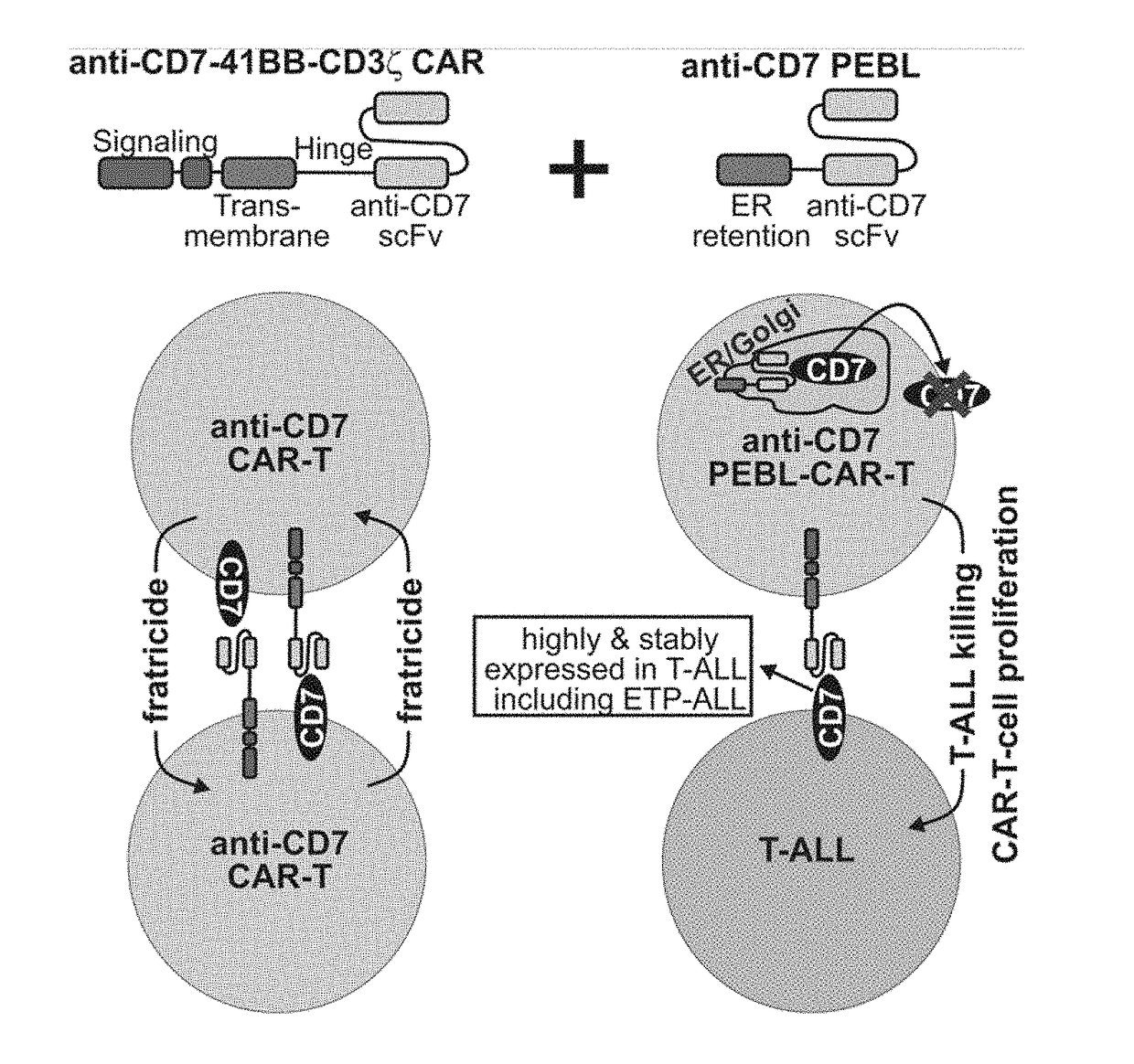
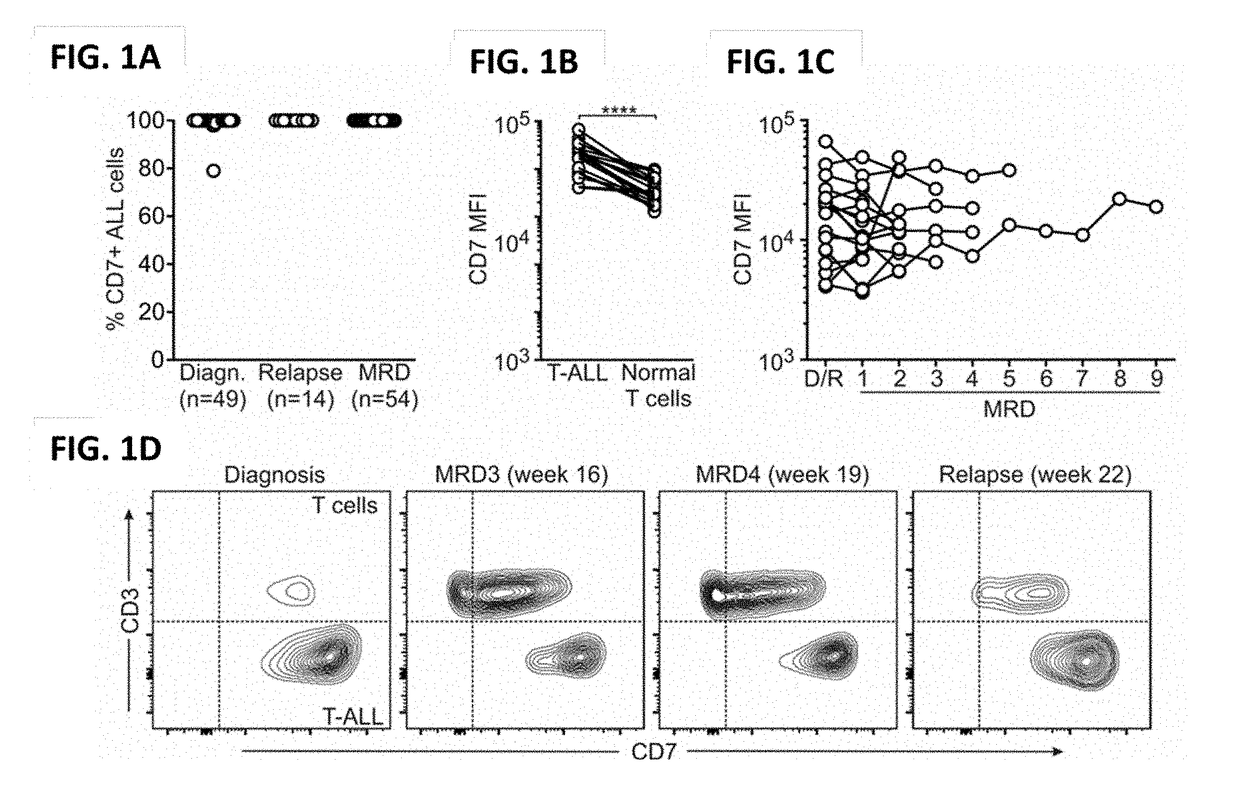
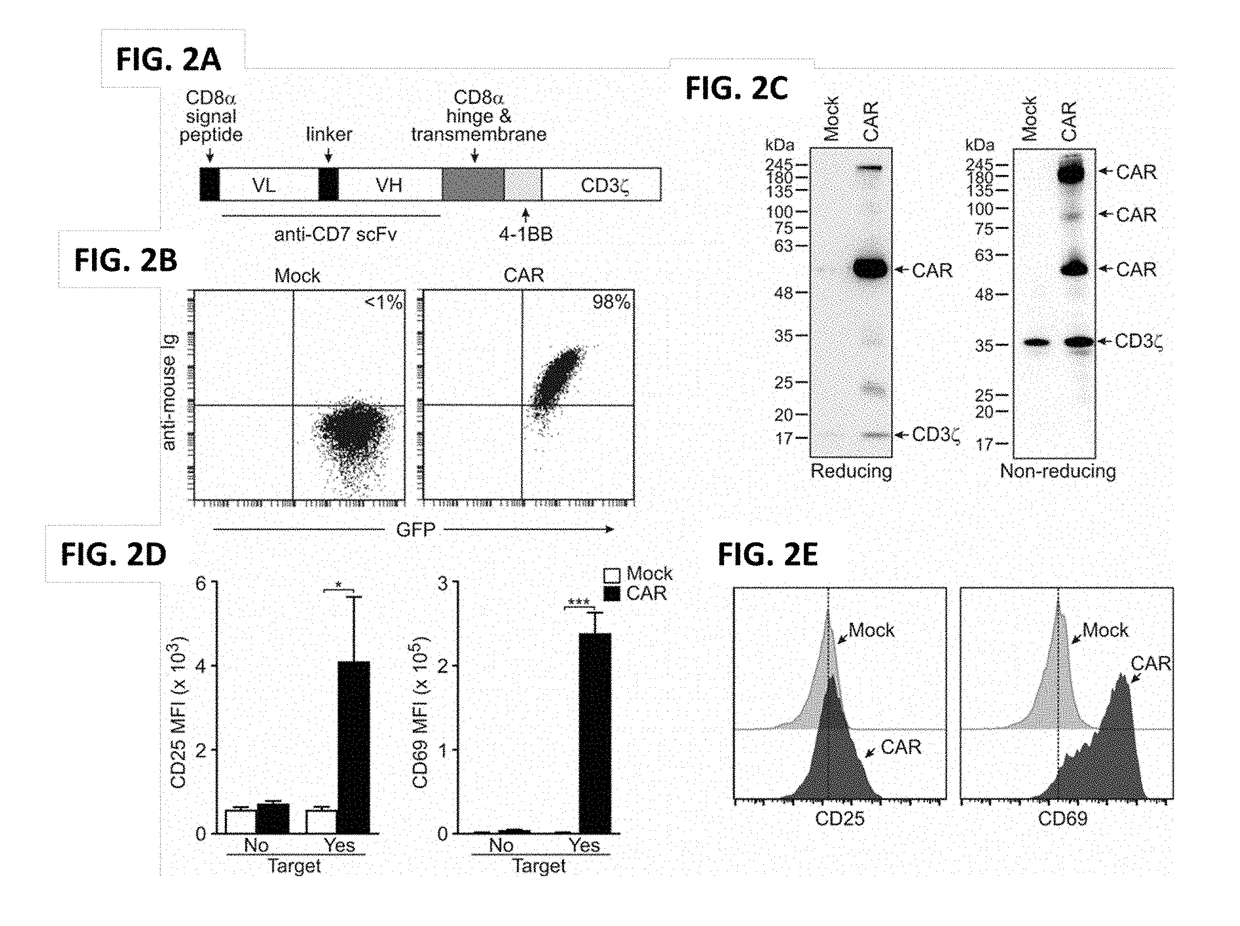
Blockade of cd7 expression and chimeric antigen receptors for immunotherapy of t-cell malignancies
ActiveUS20180148506A1Potent and durable therapeutic effectEfficient killingPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorsChimeric antigen receptor
Owner:NAT UNIV OF SINGAPORE
Genetically-modified cells comprising a modified human t cell receptor alpha constant region gene
ActiveUS20170335010A1Efficient insertionConvenient treatmentAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseFhit gene
Disclosed herein is a genetically-modified cell comprising in its genome a modified human T cell receptor alpha constant region gene, wherein the cell has reduced cell-surface expression of the endogenous T cell receptor. The present disclosure further relates to methods for producing such a genetically-modified cell, and to methods of using such a cell for treating a disease in a subject.
Owner:PRECISION BIOSCI
Tumor cell vaccines
InactiveUS20080166379A1Maximize potentialExtend your lifeBiocidePeptide/protein ingredientsCancer cellImmunogenicity
An effective cancer cell vaccine for canines has been developed. The vaccine is prepared from autologous lymphoma cells transfected with emm55. Once an animal is vaccinated, the expressed Emm55 antigen stimulates an immunogenic response to the tumor cells resulting in significantly increased survival, strong autologous and cross reactive humoral and cell mediated responses in several breeds of dogs diagnosed with later stage lymphomas.
Owner:MORPHOGENESIS
Preparation method of immune cell exosome carrying chimeric antigen receptor and application thereof
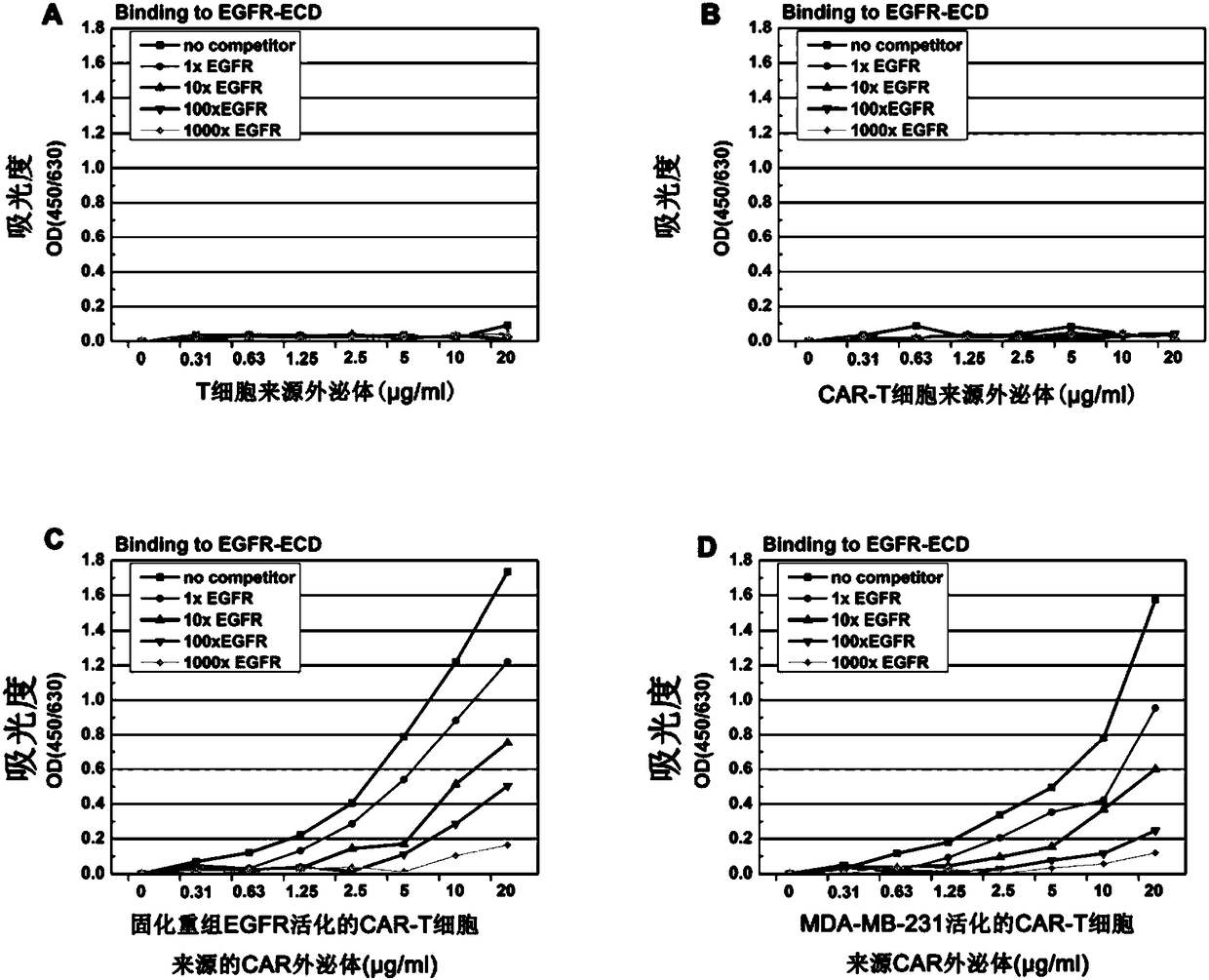
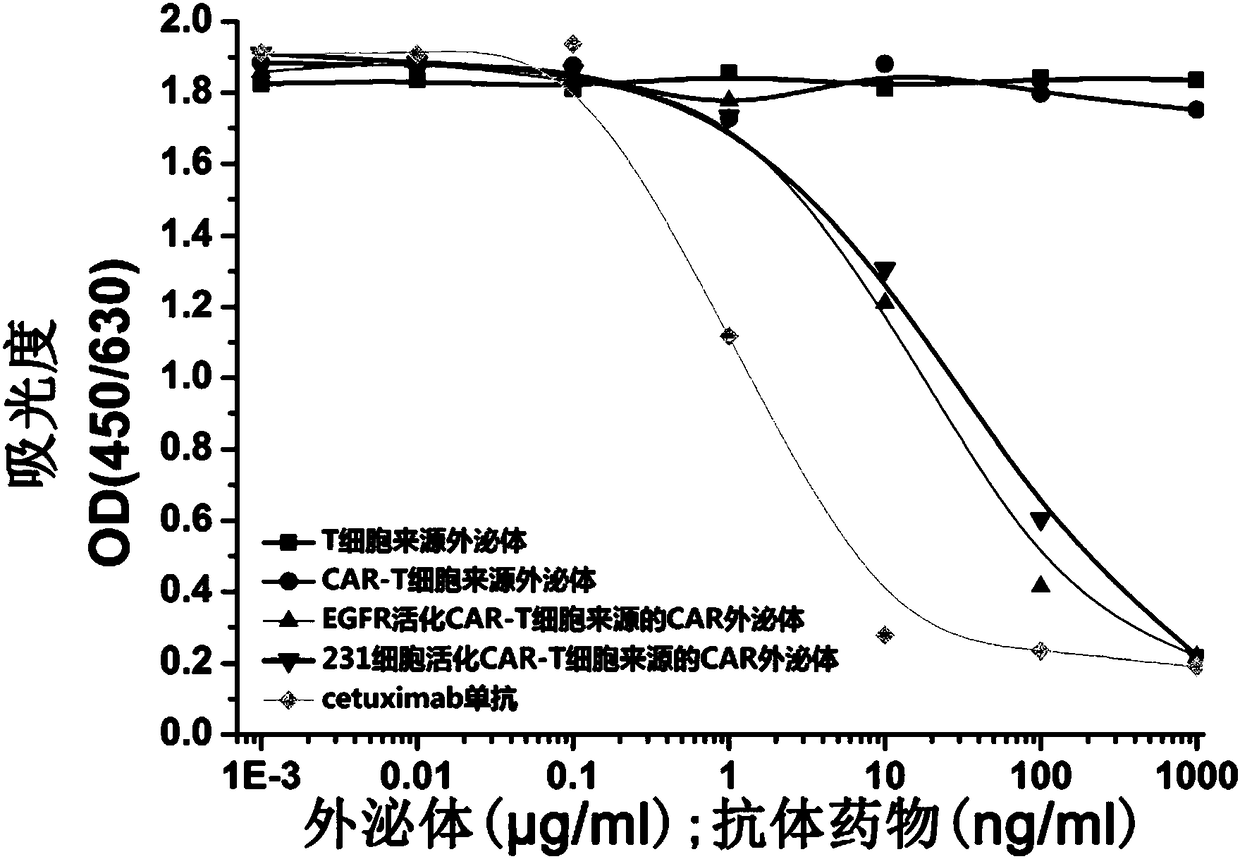
ActiveCN108315305AEnhanced tissue penetrationAbility to overcome adverse reactionsOrganic active ingredientsMammal material medical ingredientsAntigenDisease
The invention relates to the field of biological medicine, in particular to a preparation method for obtaining immune cell exosome carrying a CAR through separation. Specifically CAR immune cell is activated through specific antigen, produced exosome is further analyzed, separated, purified and enriched, and finally the immune cell exosome carrying the CAR is obtained. The exosome can be applied to the treatment of various diseases, such as cancer and severe infectious diseases, overcomes adverse reactions, such as immune inflammation storm, during CAR cell therapy, enhances the CAR tissue infiltration capacity, further has the advantages of high convenience in storage and transportation, and provides a novel strategy for the treatment of relevant diseases.
Owner:PHARCHOICE THERAPEUTICS INC
Methods for inducing a sustained immune response against a b-cell idiotype using autologous Anti-idiotypic vaccines
InactiveUS20120114634A1Eliminating and substantially reducing non-HodgkinEliminating and substantially reducingAntibody ingredientsCancer antigen ingredientsChronic lymphocytic leukemiaMantle lymphoma
The present invention relates to methods of inducing and maintaining an immune response against a B-cell idiotype in a subject using an autologous anti-idiotypic vaccine. In one embodiment, the immune response is induced and maintained for treatment of a B-cell derived malignancy selected from among non-Hodgkin's lymphoma. Hodgkin's lymphoma, chronic lymphocytic leukemia, multiple myeloma, and mantle cell lymphoma.
Owner:BIOVEST INT
Improved T cell therapy
Owner:IMMUNOTECH BIOPHARM CO LTD +3
Anti-BCMA CAR as well as expression vector and application thereof
ActiveCN111944054AEasy to removeNo toxicityImmunoglobulinsFusions for specific cell targetingTumor targetSingle-Chain Antibodies
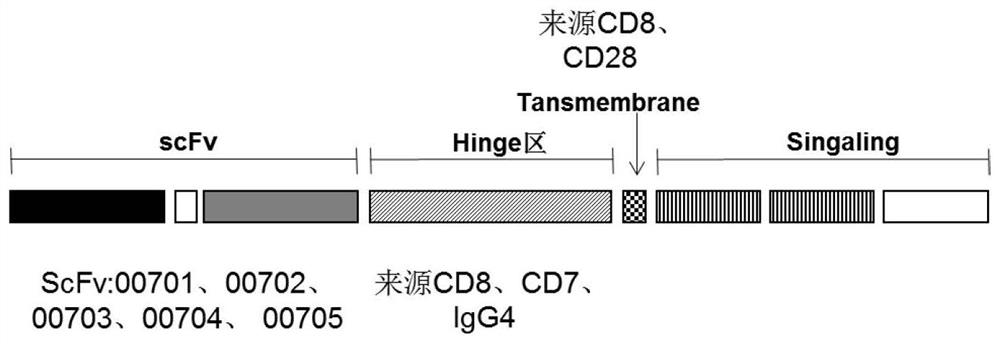
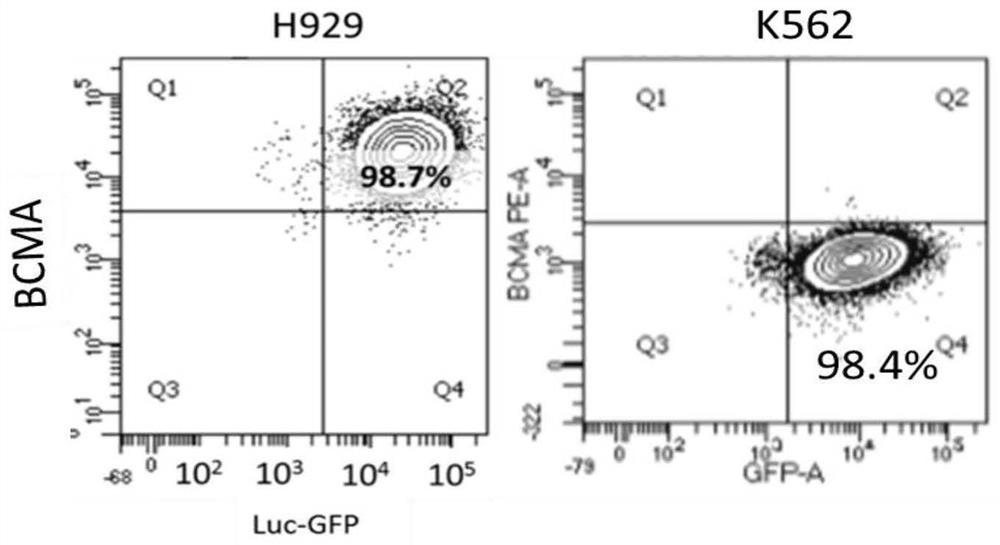
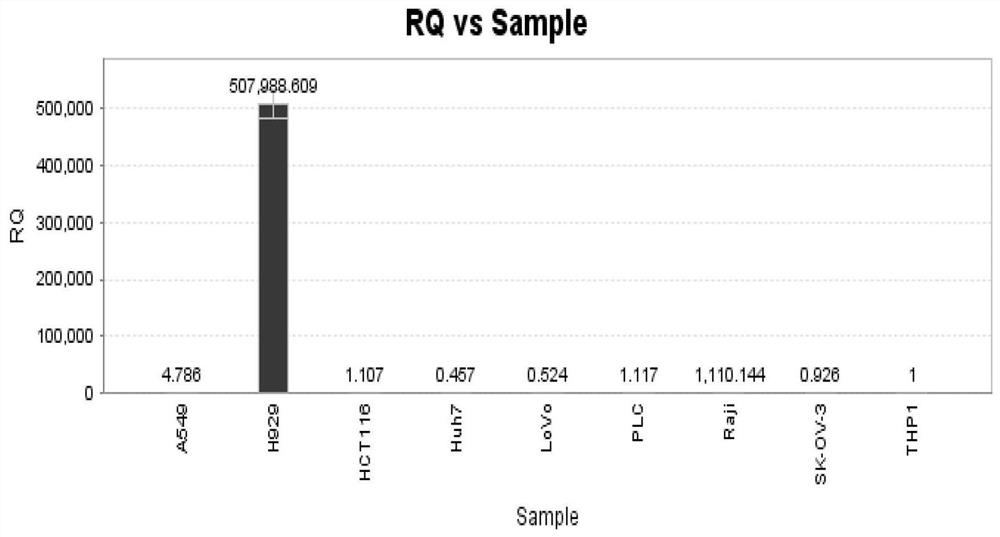
The invention belongs to the technical field of gene engineering, and particularly relates to an anti-BCMA antigen CAR and an expression vector, a CAR cell and application thereof. The CAR comprises an antigen recognition region capable of recognizing a BCMA antigen, a hinge region, a transmembrane region and an intracellular signal domain. The antigen recognition region for recognizing the BCMA antigen is an anti-BCMA single-chain antibody, wherein the heavy-chain amino acid sequence of the antigen recognition region is shown as any one of SEQ ID NO: 1-5, and the light-chain amino acid sequence of the antigen recognition region is shown as any one of SEQ ID NO: 6-10. The CAR can be stably expressed in patient-derived T lymphocytes, has better capability of removing tumor cells, and can beused for adoptive cell therapy aiming at hematological malignancies in preparation of drugs for treating hematological malignancies. The anti-BCMA antigen CAR cell not only can effectively remove tumor target cells expressing the BCMA antigen, but also has no toxic effect on negative antigen, namely tumor cells not expressing the BCMA, so that the safety is very high.
Owner:CHONGQING PRECISION BIOTECH CO LTD
Double-target chimeric antigen receptor targeting NKG2D ligand and CD19 as well as expression vector and application thereof
ActiveCN110981970AGood antitumor activityAvoid immune escapeAntibody mimetics/scaffoldsNucleic acid vectorSingle-Chain AntibodiesCD8
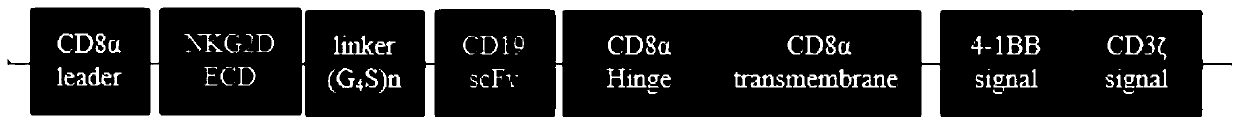
The invention discloses a double-target chimeric antigen receptor targeting an NKG2D ligand and CD19 as well as an expression vector and an application thereof, which belong to the field of tumor immune drugs. The double-target chimeric antigen receptor comprises a signal peptide, a NKG2D extracellular region of a targeted NKG2D ligand, a connecting fragment, a single-chain antibody of the targeted CD19, a lengthened CD8 alpha hinge region, a transmembrane region, a costimulatory factor and an intracellular signal peptide which are connected in sequence; the double-target chimeric antigen receptor can be used for specifically identifying tumor cells expressed by two single targets, namely a targeted NKG2D ligand or a CD19 antigen; in addition, tumor cells co-expressed by the targeting NKG2D ligand and the CD19 antigen can be recognized, the double-target CAR-T has stronger anti-tumor activity, immune escape of positive tumor cells expressed by low-abundance antigen can be avoided, andthus the recurrence risk is reduced.
Owner:华夏源细胞工程集团股份有限公司
Combination of immunotherapeutics and bisfluoroalkyl-1,4-benzodiazepinone compounds for treating lymphomas
InactiveUS20200085839A1Good curative effectPolypeptide with localisation/targeting motifOrganic active ingredientsAntigen receptorImmunotherapeutic agent
The present invention provides methods of use for compositions comprising an immunotherapeutic such as chimeric antigen receptor T cells (CAR-T cells), and specifically those that target a tumor antigen cleaved by gamma secretase, in combination with bisfluoroalkyl-1,4-benzodiazepinone compounds, including compounds of Formula (I) or prodrugs thereof;for treating lymphomas.
Owner:AYALA PHARMA INC
Chimeric antigen receptor T cell and application thereof
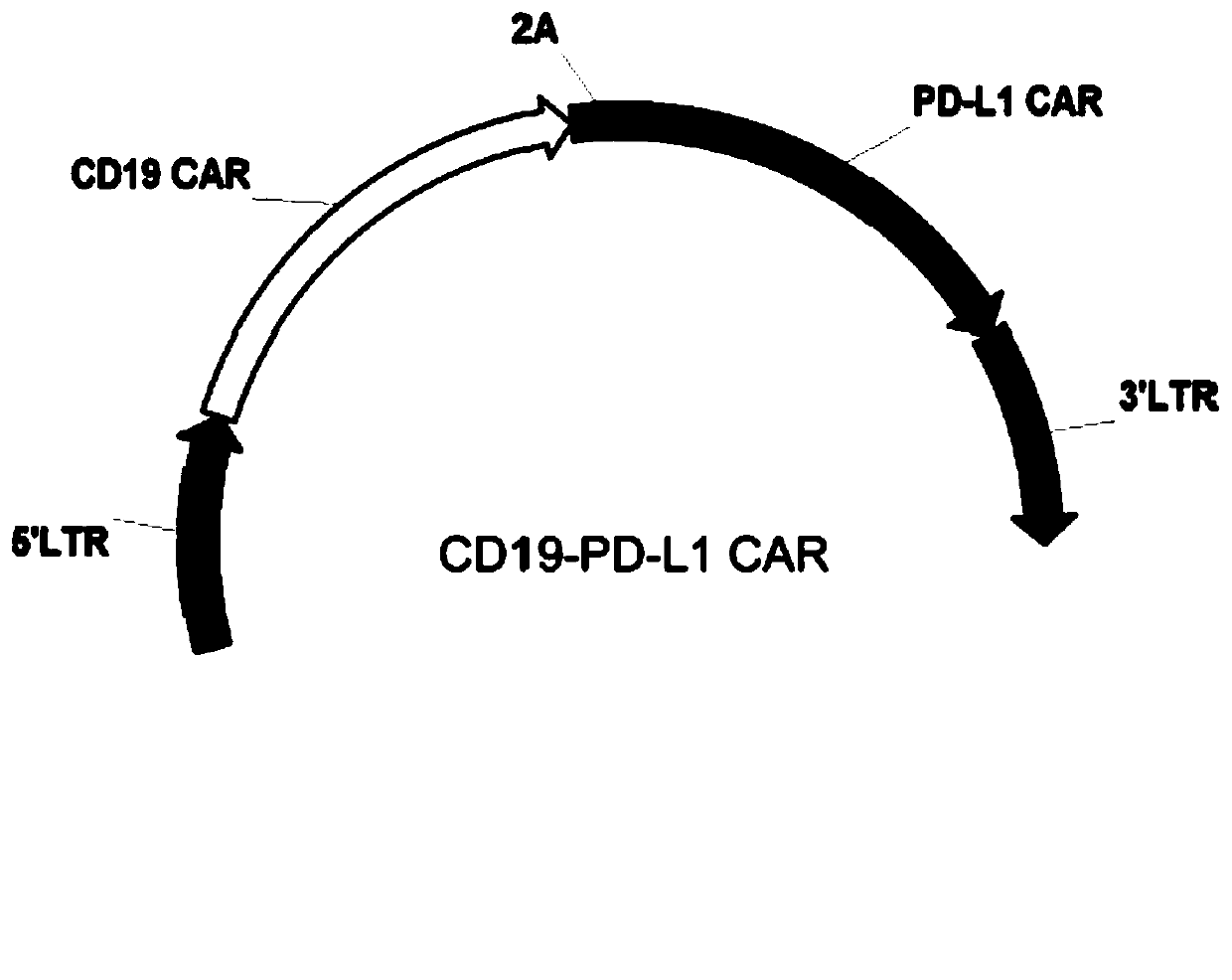
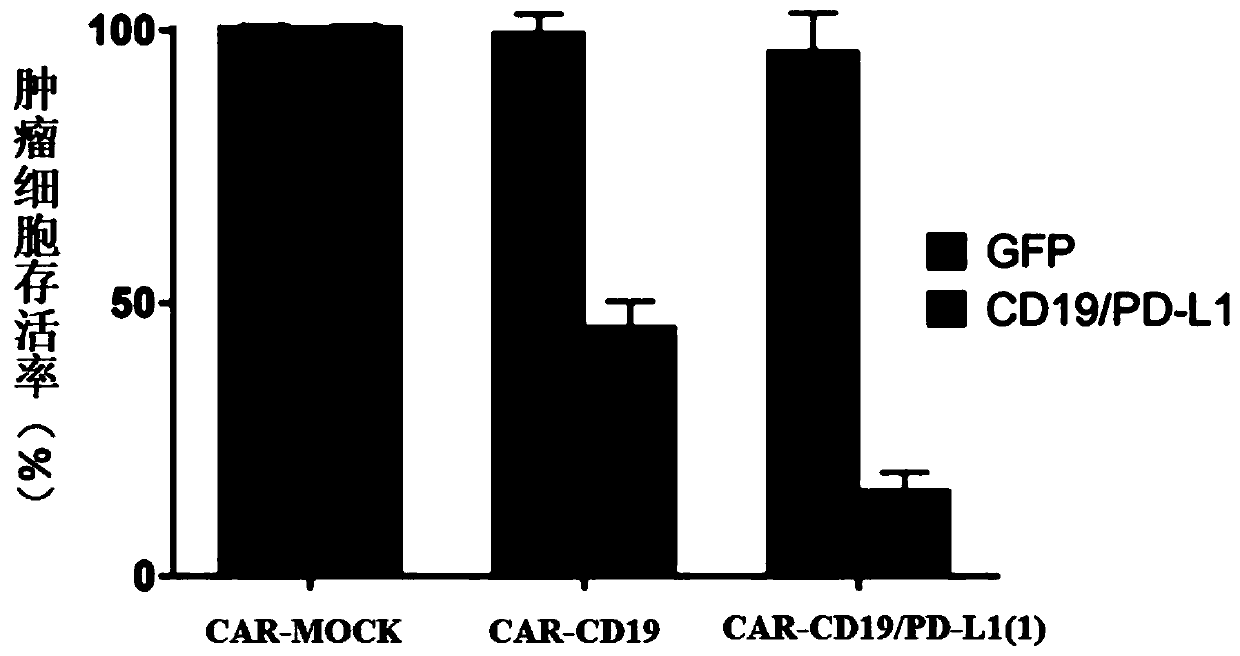
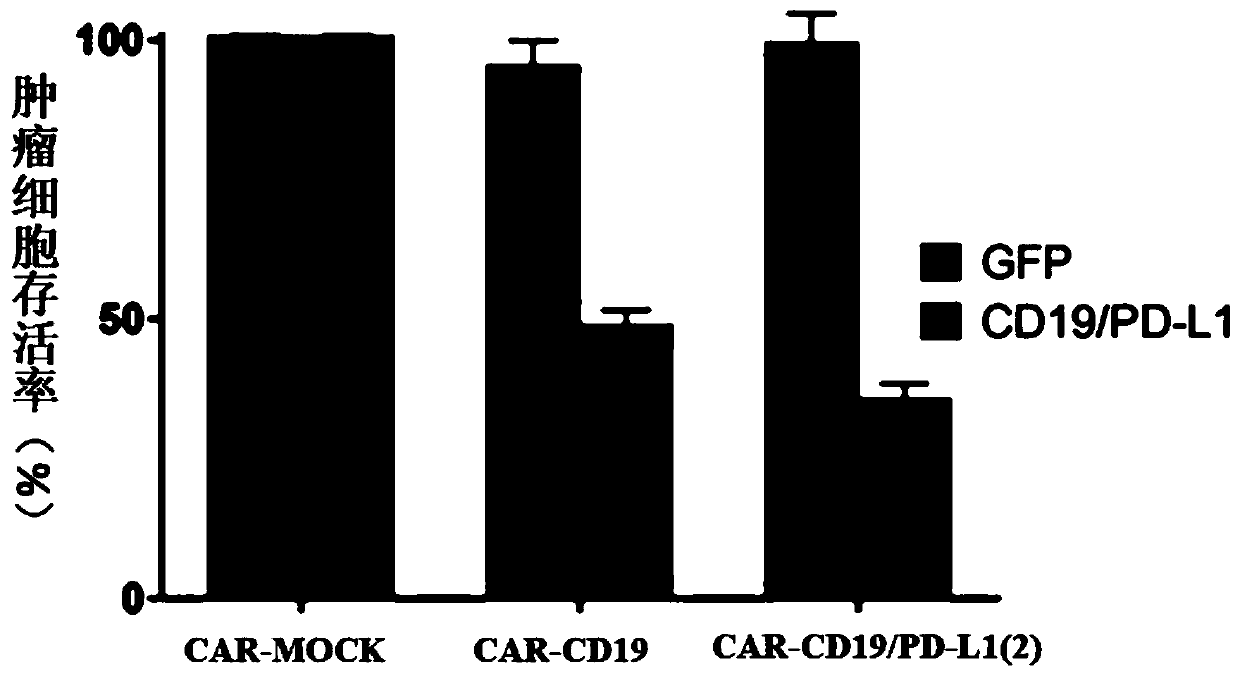
PendingCN111411085ARelief of immunosuppressionImprove anti-tumor effectAntibody mimetics/scaffoldsNucleic acid vectorAntigen receptorImmune escape
The invention provides a chimeric antigen receptor T cell and application thereof. The chimeric antigen receptor T cell expresses chimeric antigen receptors targeting CD19 and PD-L1 at the same time.The chimeric antigen receptor T cell expresses a CD19 CAR structure and a PD-L1 CAR structure at the same time, and after PD-L1 CAR is combined with PD-L1 expressed by a tumor cell, an activation signal can be transmitted intracellularly. Therefore, the chimeric antigen receptor T cell not only can recognize the tumor cells expressing CD19 in a targeted manner, but also can relieve immunosuppression of PD-1 expressed by the tumor cells on the T cells, avoid an immune escape mechanism of the tumor cells and relieve the problem of drug resistance of CAR-T treatment; and a new idea is provided for CAR-T treatment and adoptive feedback immune cell treatment.
Owner:格源致善(上海)生物科技有限公司
ANTI-FcRH5 ANTIBODIES AND IMMUNOCONJUGATES AND METHODS OF USE
InactiveUS20130089555A1Heavy metal active ingredientsPeptide/protein ingredientsHematopoietic TumorCancer research
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:GENENTECH INC
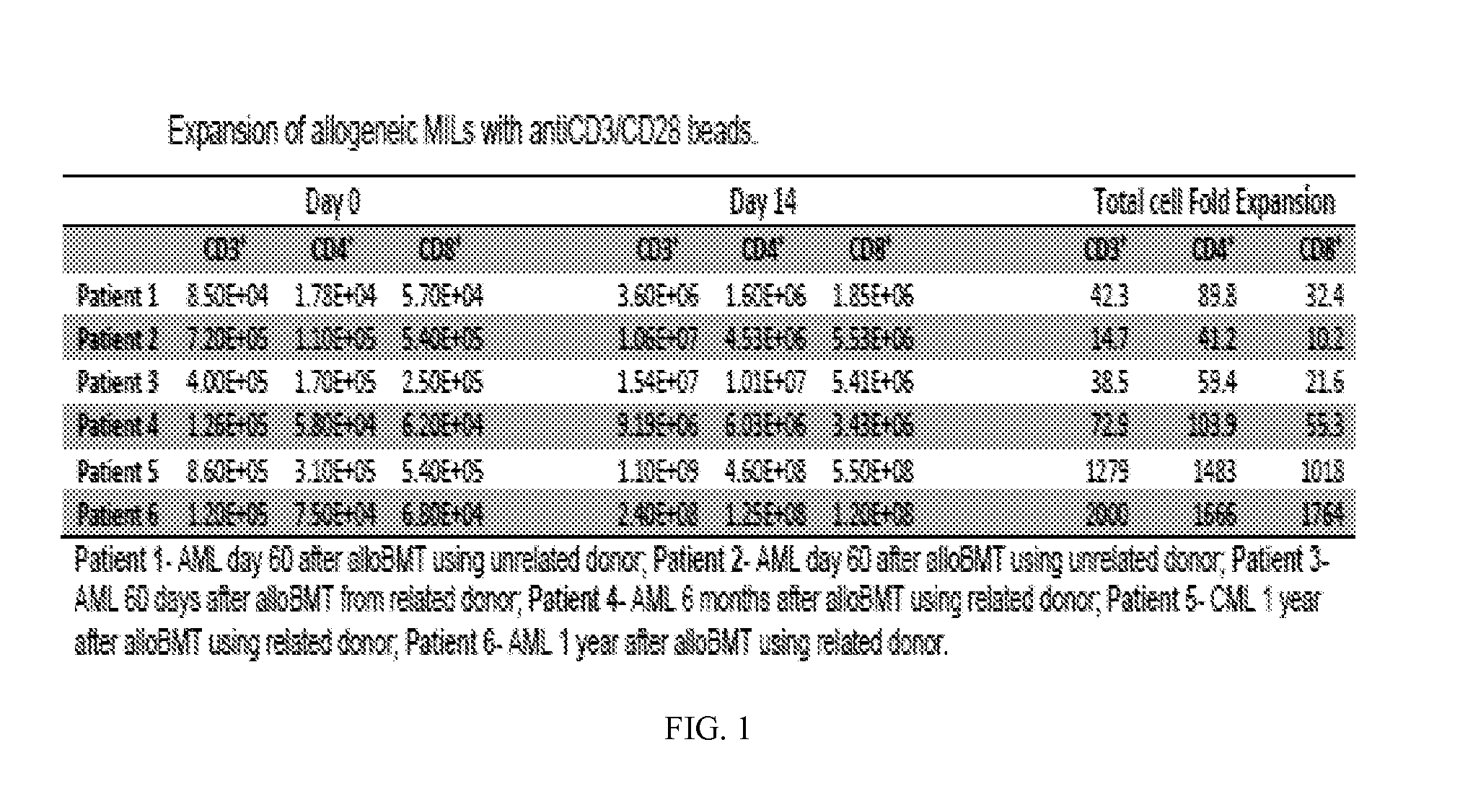
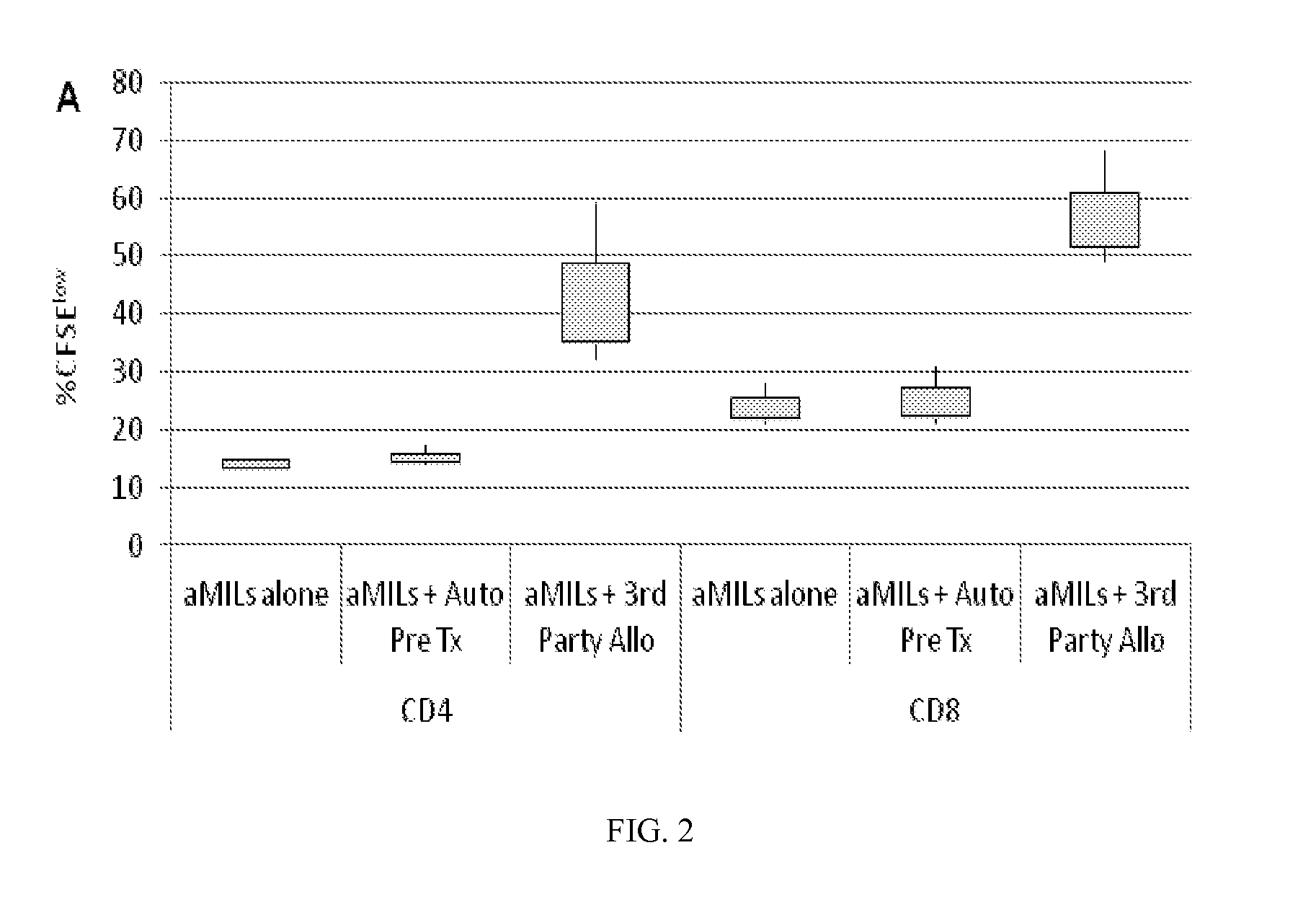
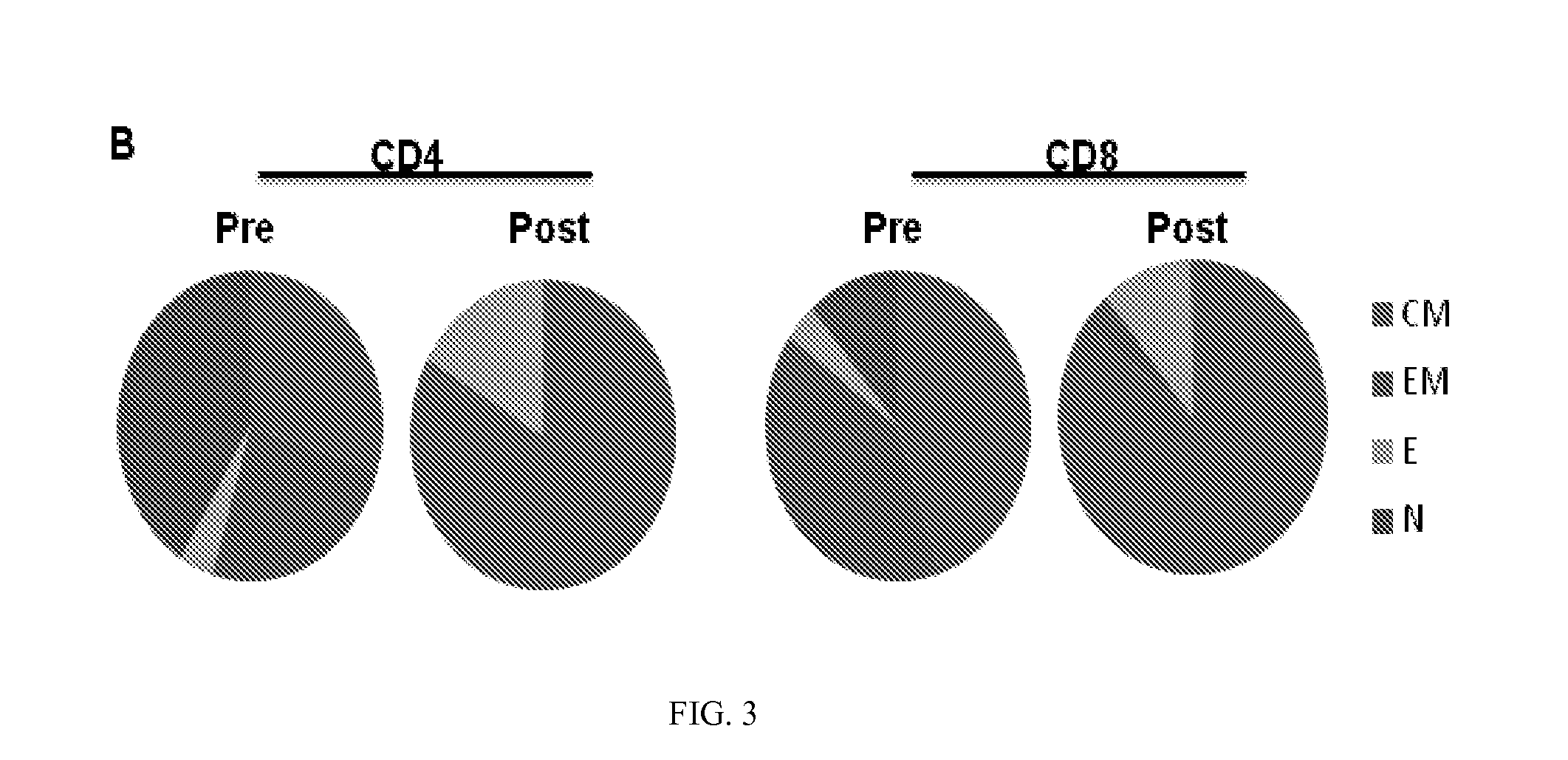
Use of Post-Transplant Cyclophosphamide Treated Allogeneic Marrow Infiltrating Lymphocytes to Augment Anti-Tumor Immunity
InactiveUS20150320798A1Reduced toxicity and mortalityStrong specificityBiocideOrganic active ingredientsTreatment fieldLymphocyte
The present invention relates to the field of cancer therapy. More specifically, the present invention provides methods and compositions useful for augmenting anti-tumor immunity. In one embodiment, a method for treating or preventing post-allogeneic transplant relapse in a subject who has received post-transplant cyclophosphamide treatment comprises the steps of (a) obtaining a bone marrow sample from the subject; (b) expanding the marrow infiltrating lymphocytes (MILs) present in the sample; and (c) administering the MILs to the subject. In a specific embodiment, the method significantly reduces the likelihood of developing GVHD.
Owner:WINDMIL THERAPEUTICS INC
Modified natural killer cells and natural killer cell lines having increased cytotoxicity
ActiveUS20170157230A1High cytotoxic activityGenetically modified cellsBoron compound active ingredientsNatural Killer Cell Inhibitory ReceptorsNatural killer cell
NK cells and NK cell lines are modified to increase cytotoxicity, wherein the cells and compositions thereof have a use in the treatment of cancer. Production of modified NK cells and NK cell lines is via genetic modification to remove checkpoint inhibitory receptor expression and / or add mutant (variant) TRAIL ligand expression.
Owner:ONK THERAPEUTICS LTD
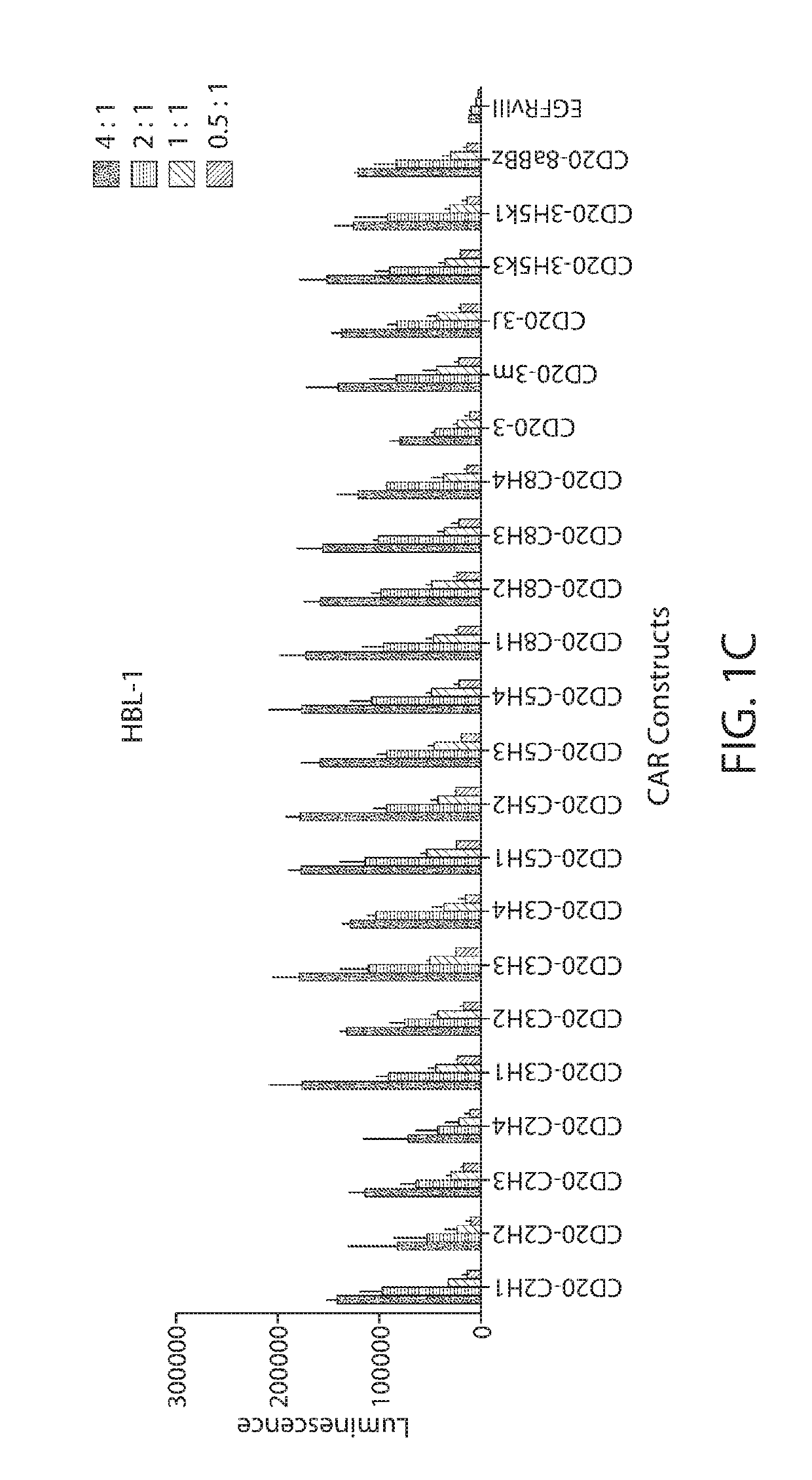
Nucleic acid molecules encoding chimeric antigen receptors comprising a CD20 binding domain
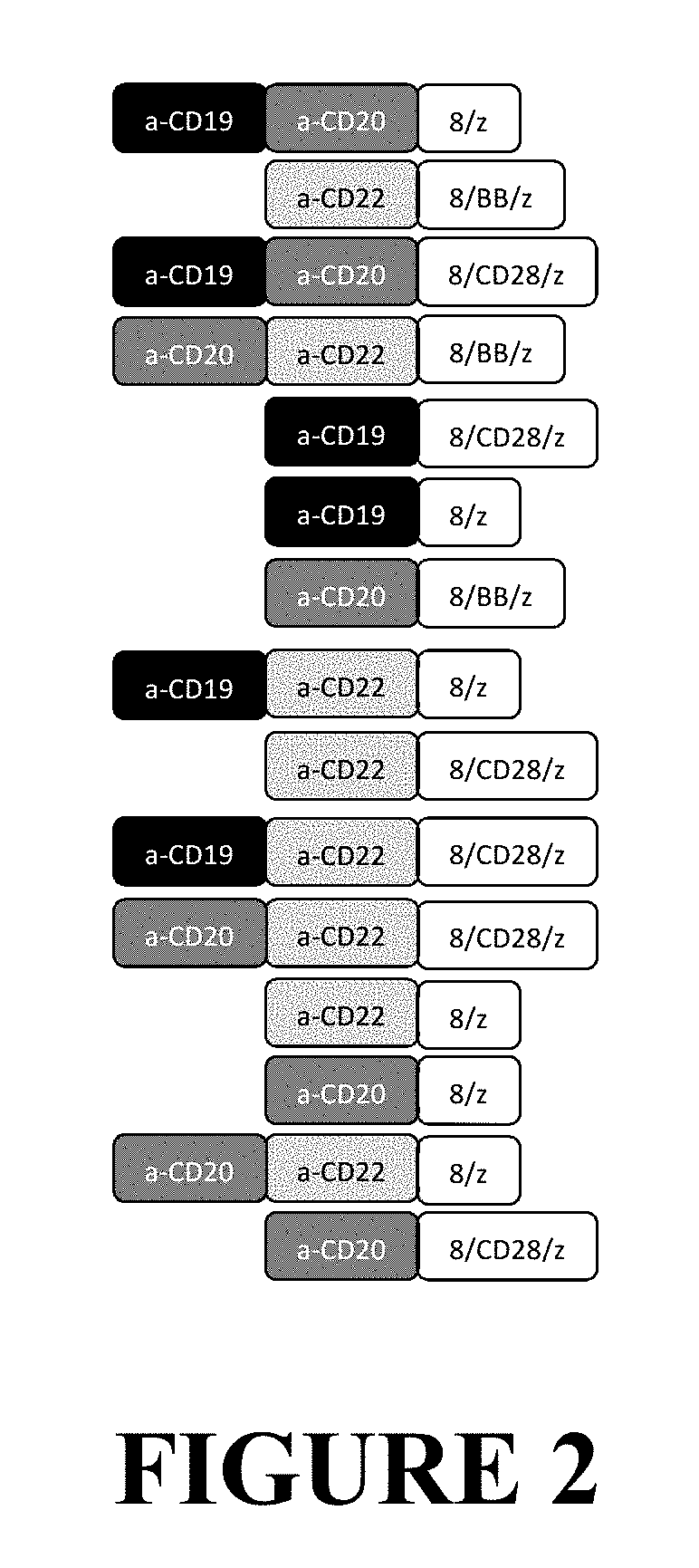
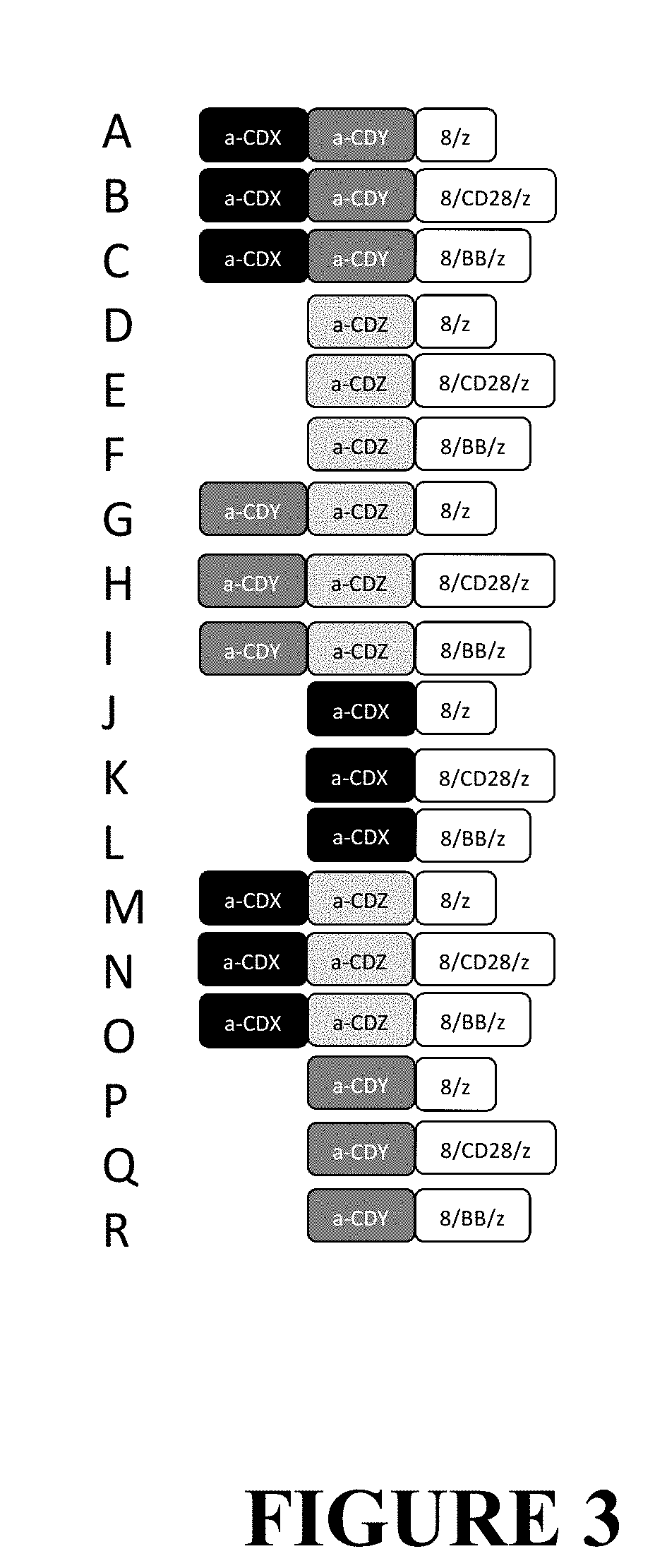
ActiveUS10525083B2Polypeptide with localisation/targeting motifImmunoglobulin superfamilyCD20Disease
The invention provides compositions and methods for treating diseases associated with expression of CD20 or CD22. The invention also relates to chimeric antigen receptor (CAR) specific to CD20 or CD22, vectors encoding the same, and recombinant T or natural killer (NK) cells comprising the CD20 CAR or CD22 CAR. The invention also includes methods of administering a genetically modified T cell or NK cell expressing a CAR that comprises a CD20 or CD22 binding domain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
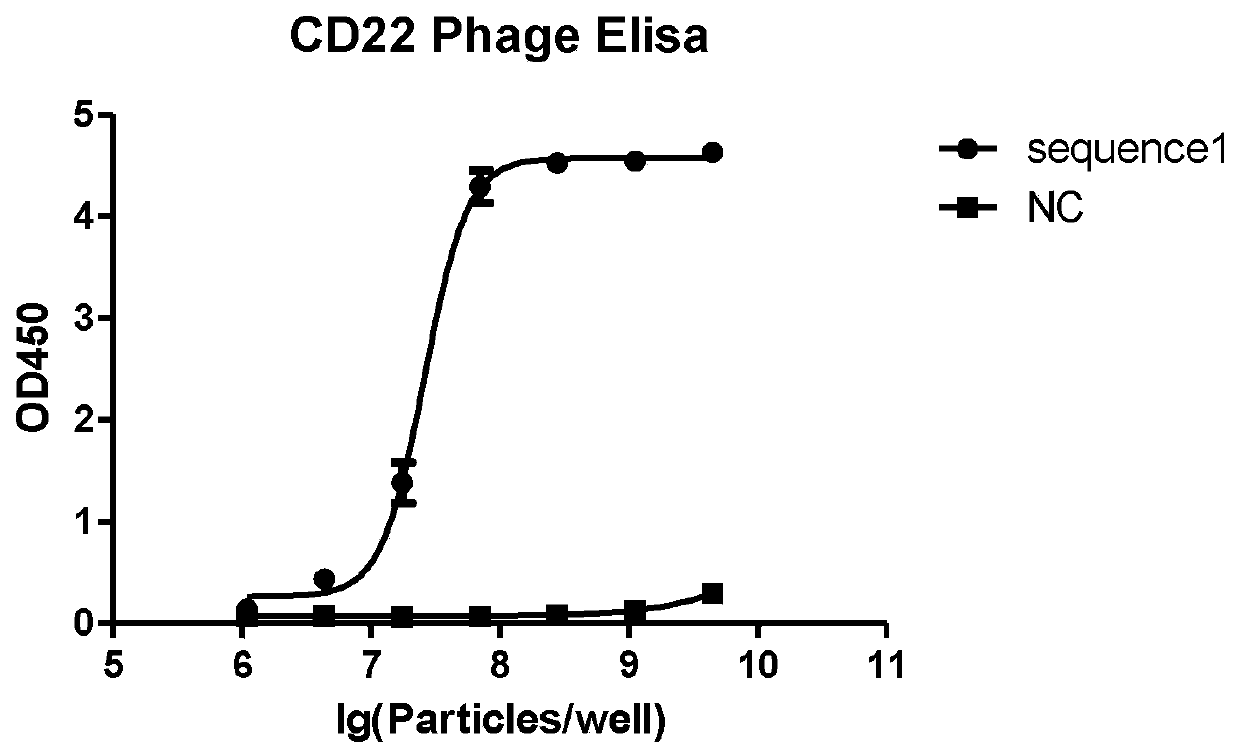
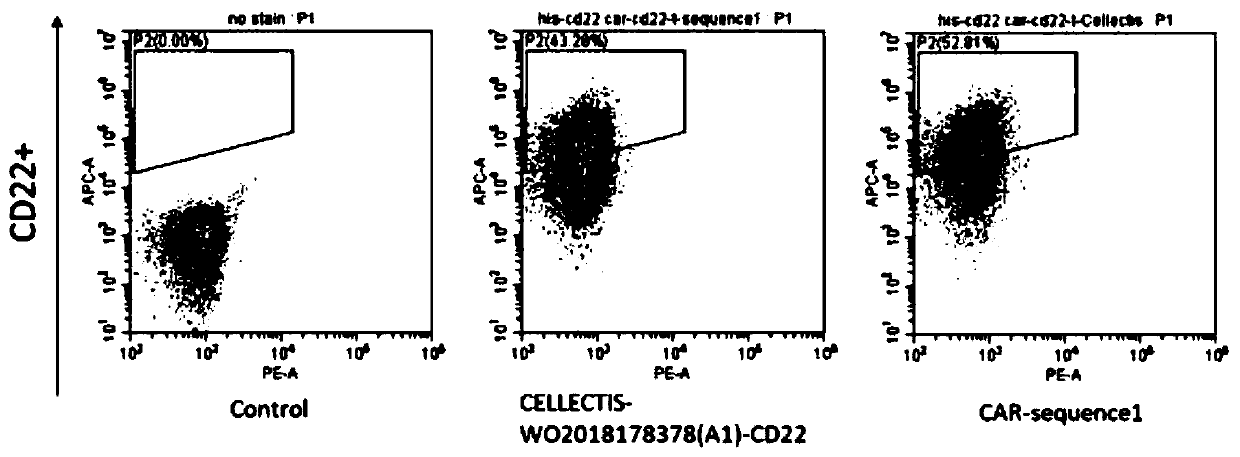
CD22 protein targeting antibody, chimeric antigen receptor and drug
ActiveCN111484562ALittle side effectsIncrease lethalityPolypeptide with localisation/targeting motifImmunoglobulin superfamilyProtein targetAntigen binding
The invention provides a CD22 protein targeting antibody, a chimeric antigen receptor and a drug, and relates to the technical field of cellular immunotherapy. The chimeric antigen receptor has an antigen binding domain aiming at a tumor surface antigen CD22, and the amino acid sequence of a heavy chain variable region of the antigen binding domain is shown as SEQ ID NO.3; the amino acid sequenceof a light chain variable region of the antigen binding domain is shown as SEQ ID NO. 7. The chimeric antigen receptor can target a CD22 protein; T cells prepared from the chimeric antigen receptor can specifically act on CD22 positive target cells, have very good specificity and sustainability, are high in killing capacity and small in side effect on healthy tissues, and can be used for preparingmedicines for treating or preventing CD22 positive tumors.
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV
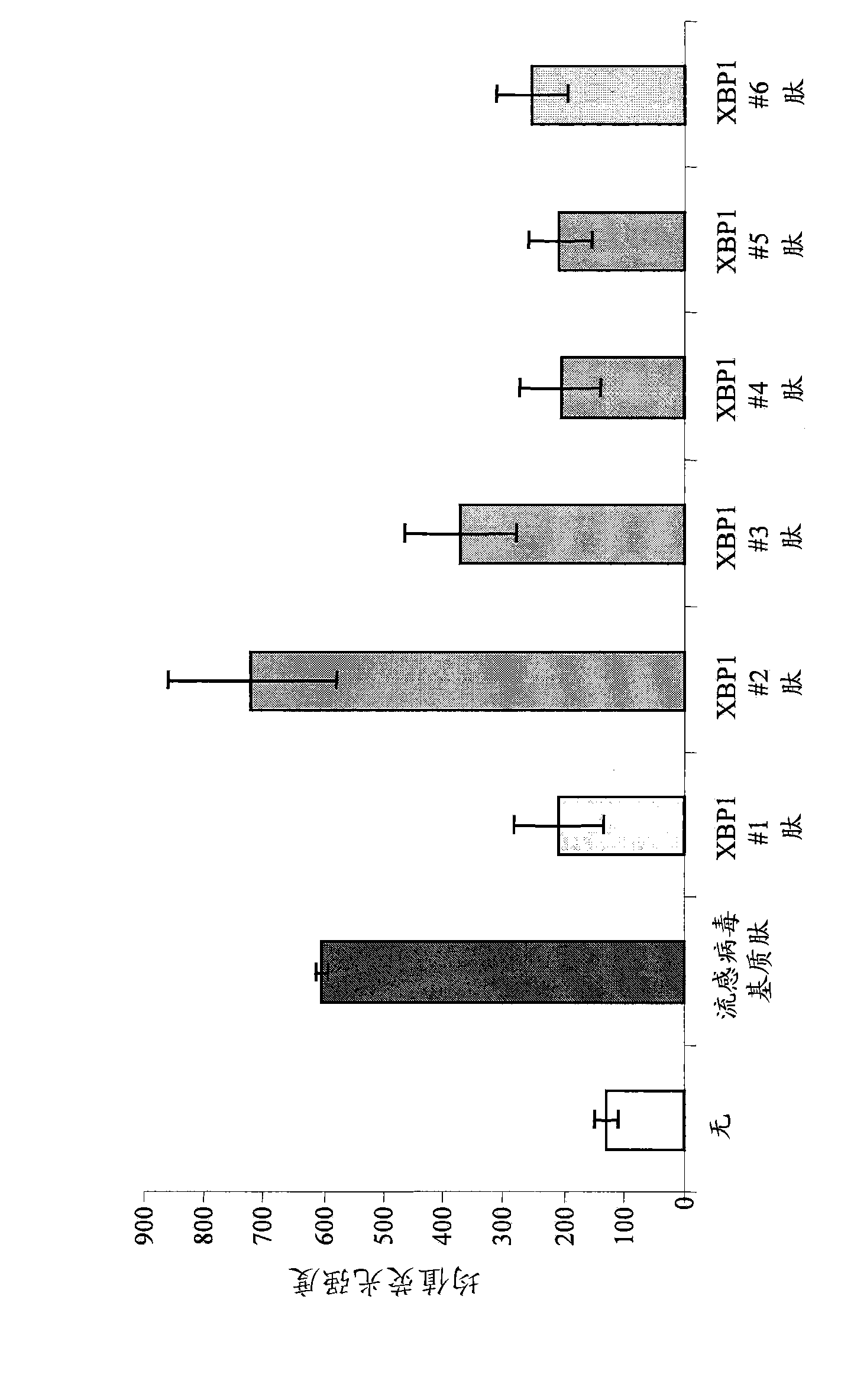
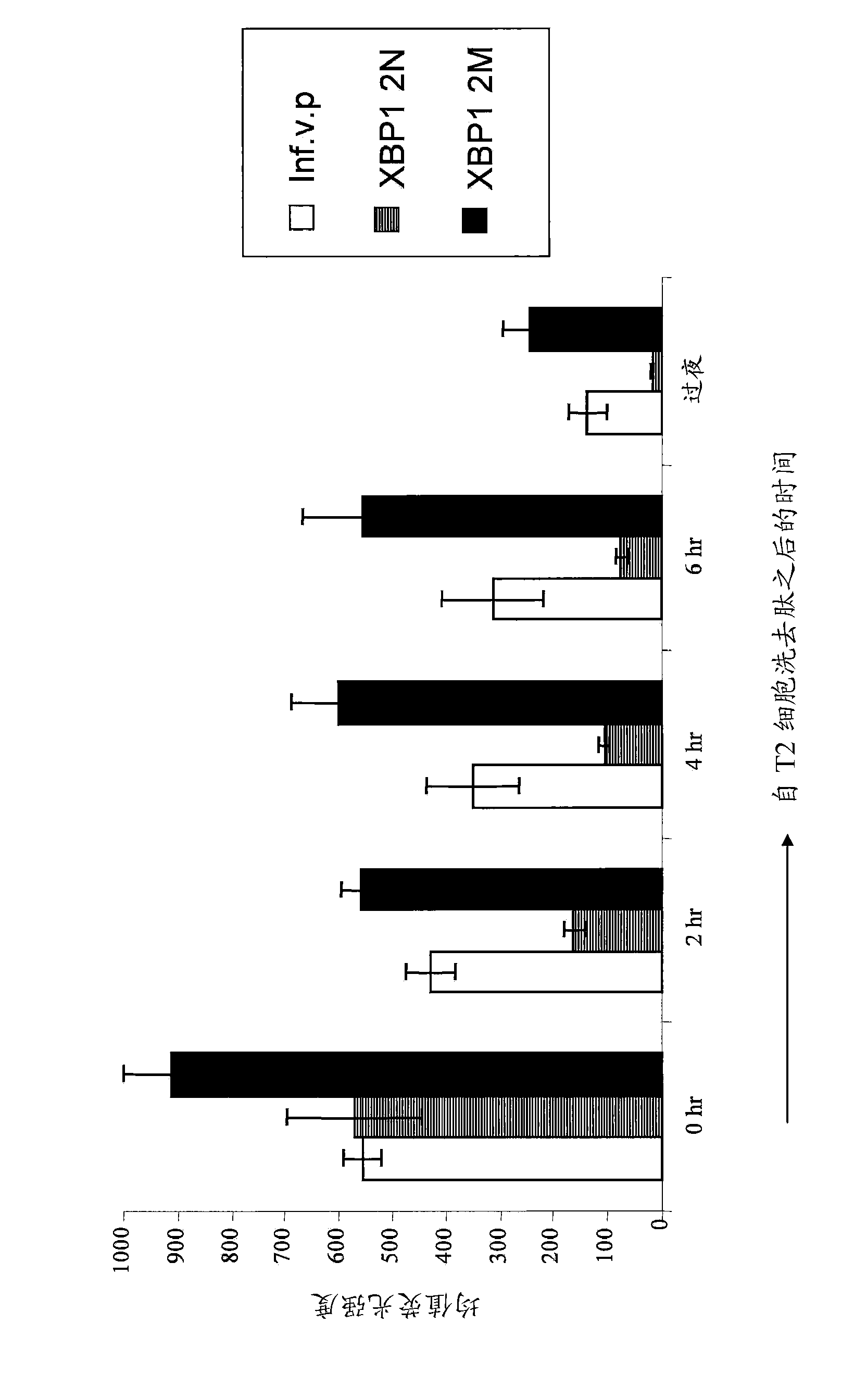
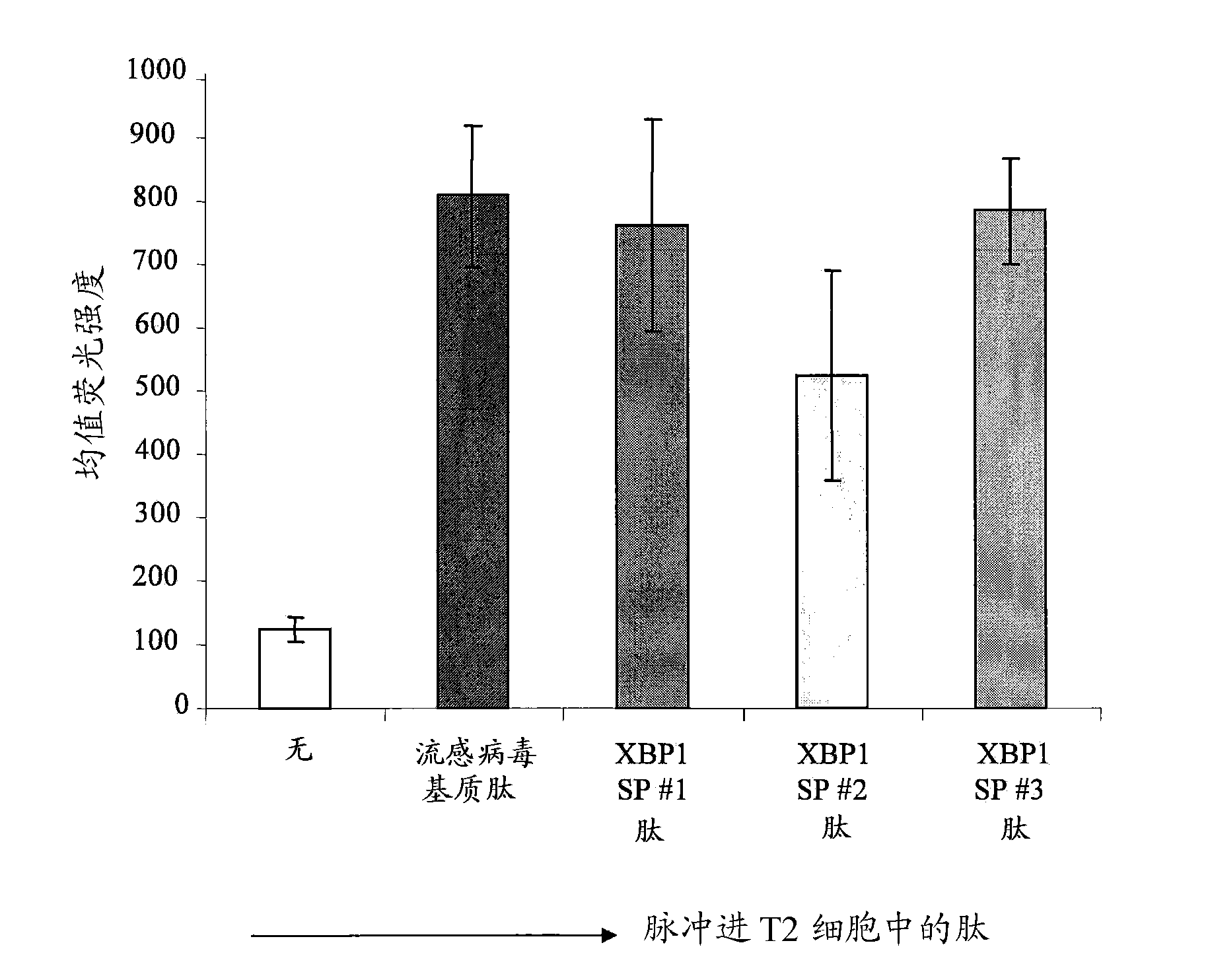
XBP1, CD138, and CS1 peptides
The invention discloses features, inter alia, immunogenic XBP1-, CD138-, and CS1-derived peptides (and pharmaceutical compositions thereof). The peptides can be used in a variety of methods such as methods for inducing an immune response, methods for producing an antibody, and methods for treating a cancer (e.g., a plasma cell disorder such as multiple myeloma or Waldenstrom's macroglobulinemia). The peptides can also be included in MHC molecule multimer compositions and used in, e.g., methods for detecting a T cell in a population of cells.
Owner:DANA FARBER CANCER INST INC
A Composition, A Treatment Method and An Application Thereof
InactiveUS20190015458A1Good effectImprove efficiencyColon cancer vaccineProtozoa material medical ingredientsMelanomaTreatment field
The present invention relates to the field of treatment of tumor, and especially to a composition comprising a plasmodium, a treatment method and an application thereof. The composition of the present invention has therapeutic effects on colorectal carcinoma, lung carcinoma, breast carcinoma, gastric carcinoma and hepatic carcinoma etc., can inhibit the growth of tumor and prolong the life of the tumor patients, whereas has no therapeutic effect on melanoma and lymphoma; meanwhile, the present invention describes that the long-term plasmodium infection has better therapeutic effect on tumors, and the plasmodium immunotherapy of the present invention does not take the fever time as a course standard when treating tumors, but should be used to extend the duration of plasmodium infection as much as possible until the progression of tumors can be controlled under the premise of protecting the organ functions and life safety of the patients.
Owner:BLUE ELEGANT BIOTECH CO LTD
Double-target chimeric antigen receptor targeting CD19 and CD20 as well as expression vector and application thereof
ActiveCN110981971AGood antitumor activityAvoid immune escapeAntibody mimetics/scaffoldsNucleic acid vectorCD20Single-Chain Antibodies
The invention discloses a CD19 and CD20 targeting double-target chimeric antigen receptor and an expression vector and an application thereof, which belong to the field of tumor immune drugs. The double-target chimeric antigen receptor comprises a signal peptide, a single-chain antibody targeting CD19, a single-chain antibody targeting CD20, a CD8 alpha hinge region, a transmembrane region, a costimulatory factor and an intracellular signal peptide which are connected in sequence. The double-target chimeric antigen receptor can be used for specifically identifying tumor cells expressed by twosingle targets, namely a CD19 antigen and a CD20 antigen, can be used for identifying tumor cells co-expressed by CD19 antigen and CD20 antigen, the double-target CAR-T has stronger anti-tumor activity, and can prevent positive tumor cells expressed by low-abundance antigen from generating immune escape, so that the recurrence risk is reduced.
Owner:华夏源细胞工程集团股份有限公司
Bispecific chimeric antigen receptor for treating hematologic tumor complicated with HIV infection, gene thereof, and construction method and application of gene
ActiveCN111196858AAchieve the effect of treating two diseases at the same timeEasy to removeVirusesAntibody mimetics/scaffoldsSingle-Chain AntibodiesAntiendomysial antibodies
The invention discloses a construction method and an application of a recombinant gene of a bispecific chimeric antigen receptor for treating HIV infection complicated with blood tumor. The chimeric antigen receptor is formed by sequentially connecting a signal peptide, an HIV gp120 antigen specific single-chain antibody and an anti-CD19 single-chain antibody and then sequentially connecting witha CD28 transmembrane region, a CD28 intracellular structural domain (ICD), a 4-1BB costimulatory structural domain and a CD3zeta intracellular signal transduction structural domain in series, or the chimeric antigen receptor comprises: first CAR composed of the signal peptide, the chimeric antigen receptor, the HIV gp120 antigen specific single-chain antibody, the CD28 transmembrane region, the CD28-ICD, the anti-CD19 single-chain antibody, the CD8 transmembrane region, the CD28-ICD, the 4-1BB costimulatory structural domain and the CD3zeta intracellular signal transduction domain; and secondCAR composed of and the signal peptide, the anti-CD19 single-chain antibody, the CD8 transmembrane region in parallel, connecting the CD28-ICD, the 4-1BB costimulatory structural domain and the CD3zeta intracellular signal transduction domain, wherein the first CAR and the second CAR are sequentially connected in parallel.
Owner:WUHAN UNIV OF SCI & TECH
Cancer antigen targets and uses thereof
ActiveUS20180348227A1Avoid problemsReduces and eradicates tumor burdenAntibody mimetics/scaffoldsGenetic material ingredientsCancer antigenSubject matter
The presently disclosed subject matter provides methods and compositions for treating myeloid disorders (e.g., acute myeloid leukemia (AML)). It relates to immunoresponsive cells bearing antigen recognizing receptors (e.g., chimeric antigen receptors (CARs)) targeting AML-specific antigens.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Compositions and methods for t cell engineering
PendingCN113383071AVirusesPeptide/protein ingredientsNatural Killer Cell Inhibitory ReceptorsAntigen binding
The disclosure relates to an engineered immune cell and use thereof. The engineered immune cell provided in the disclosure comprises a CAR or engineered TCR, which CAR or engineered TCR can comprise a first antigen binding domain and a second antigen binding domain. The engineered immune cells, when administered into a subject, can inhibit the host immune cells such as T cells and / or NK cells and enhance the survival and persistence of the engineered immune cells in vivo, thereby exhibiting more effective tumor killing activity.
Owner:GRACELL BIOTECH SHANGHAI CO LTD +1
CD19 and CD30 double-target chimeric antigen receptor and application thereof
ActiveCN111848822AStrong targeting activityEfficient targetingMicroorganism based processesImmunoglobulinsDiseaseSingle-Chain Antibodies
The invention provides a CD19 and CD30 double-target chimeric antigen receptor and an application thereof. The chimeric antigen receptor comprises an antigen binding structural domain, a transmembranestructural domain and a signal transduction structural domain, wherein the antigen binding structural domain comprises an anti-CD19 single-chain antibody and an anti-CD30 single-chain antibody. The anti-CD19 and anti-CD30 double-target chimeric antigen receptor disclosed by the invention has targeting activity on CD19 positive and / or CD30 positive cells, the T cell for expressing the anti-CD19 and anti-CD30 double-target chimeric antigen receptor has a killing effect on tumor cells with low CD19 antigen expression quantity or no CD19 antigen expression quantity and tumor cells with low CD30 antigen expression quantity or no CD19 antigen expression quantity, so that the immune escape phenomenon is avoided, and the possibility of disease recurrence is reduced.
Owner:GUANGDONG ZHAOTAI INVIVO BIOMEDICINE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com